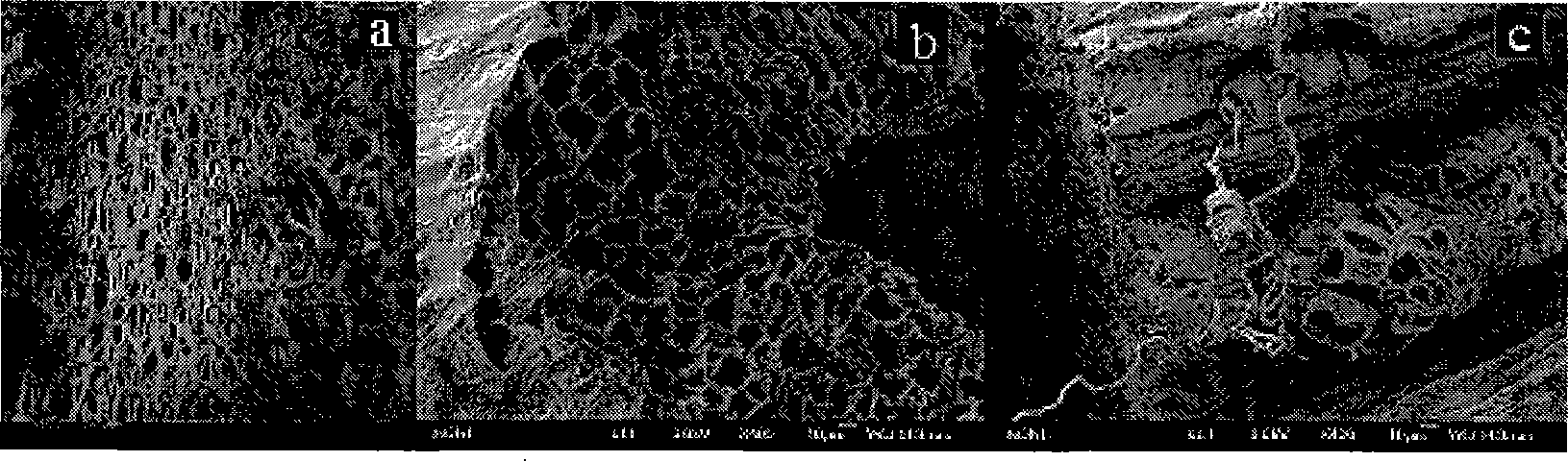
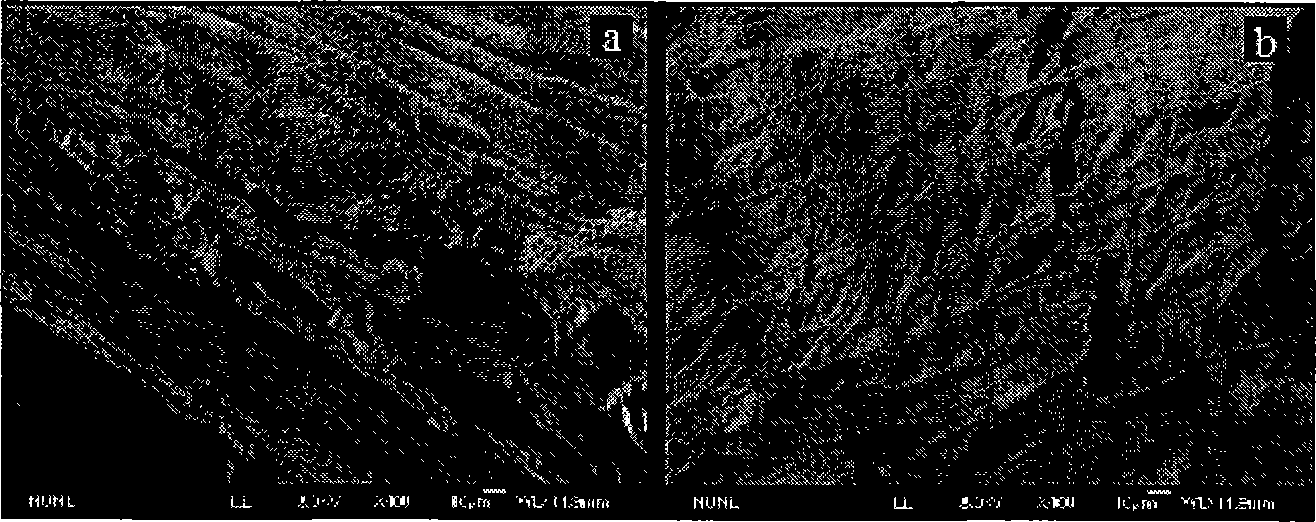
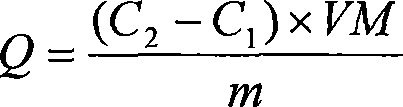Alkalization modifying method of luffa and use thereof
A technology of loofah and modification, applied in chemical instruments and methods, other chemical processes, adsorption water/sewage treatment, etc., can solve the problems of high development cost, secondary pollution, environmental pollution, etc., achieve low price and increase income , Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: 1.0 gram of the natural loofah of cutting fine and 20 grams of mass percent concentration are mixed with NaOH ethanol solution of 30%, alkalized at 25 ℃ for 24h, obtain mixed system, transfer to three-necked flask then, in Reflux for 3 hours in a constant temperature water bath at 80°C for thermal alkalization treatment. Then the alkalized sample was washed 4 times with deionized water until the pH value was 7, and then dried at 70°C after suction filtration to obtain the alkali-treated loofah.
Embodiment 2
[0031] Embodiment 2: Atomic emission spectrometry determines the adsorption capacity of alkalized modified loofah to mixed metal ions
[0032] (1) Get 1.5g of the alkali-treated loofah obtained in Example 1, transfer it to a three-necked bottle, add 150mL of metal ion mixed solution, add an appropriate amount of HCl or HAc dropwise, adjust the pH value to about 3, and react at 70°C for 6 hours . The loofah was washed three times with deionized water until the pH was equal to 7, and then dried at 80°C for atomic emission spectrometry. (2) In the adsorption experiment, three different mixed solutions of metal ions were used:
[0033] a) contains Ca 2+ 、Na + 、Cu 2+ , Mn 2+ 、Ba 2+ , Fe 3+ , Zn 2+ 、Cd 2+ Hydrochloride solution (the concentration of each metal ion is about 0.01M)
[0034] b) Contains ZnCl 2 , Pb(Ac) 2 , CdCl 2 The mixed solution (the concentration of each metal ion is about 0.005M)
[0035] c) Contains Pb(Ac) 2 , Co(NO 3 ) 2 , AgNO 3 The test resul...
Embodiment 3
[0047] Embodiment 3: Atomic Absorption Spectroscopy (AAS) investigates its single ion Zn 2+ or Cu 2+ adsorption capacity
[0048] (1) Weigh 2 parts of the loofah of 0.4g embodiment 1 gain respectively, place in 100mL beaker, add the Zn that contains 5mmol respectively 2+ and Cu 2+ 40mL of a single metal ion solution, adjust the pH value to 4-6, and let it stand for adsorption at room temperature for 24 hours.
[0049] (2) Collect the filtrate in the beaker into a 100 mL volumetric flask, and wash the alkalized modified loofah twice with deionized water, 10 mL each time, and put the filtrate into the volumetric flask. Use atomic absorption spectrometry to measure the residual ion concentration, and then convert it into the adsorption amount of alkali-treated loofah on metal ions.
[0050] When using atomic absorption spectrometry to determine the concentration of metal ions, proceed through the following steps:
[0051] (1) Prepare standard solutions, measure the absorbanc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com