Compound having anti-fouling activity as well as extraction method and use thereof
An extraction method and anti-fouling technology, applied in the field of isoquinoline compounds, can solve the problems of poisoning attached organisms, destroying ecological balance, polluting the environment, etc., achieving the effects of good lipophilicity, simple structure, and avoiding dissolution and loss.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 compound
[0029] Source of materials: The sea paint samples were collected from Dongzhai Port, Hainan. The species was identified by Professor Lin Yiming, School of Life Sciences, Xiamen University, and the specimens were deposited in the Institute of Ecology, School of Life Sciences, Xiamen University.
[0030] Extraction and separation: crush the dried sea lacquer root into particles with a particle size of 0.1-0.5 cm, soak the crushed root in 95% ethanol for 48 hours, and repeat 3 times; filter the extract and evaporate the solvent with a rotary evaporator. into an extract; the extract is dispersed in water (200ml water per 100g extract) and extracted with ethyl acetate (1:1 with water volume ratio), and then the ethyl acetate is evaporated with a rotary evaporator to obtain a crude product; the crude product is used Petroleum ether: acetone was purified by silica gel column chromatography (4:1) as the eluent, recrystallized in aceton...
Embodiment 2
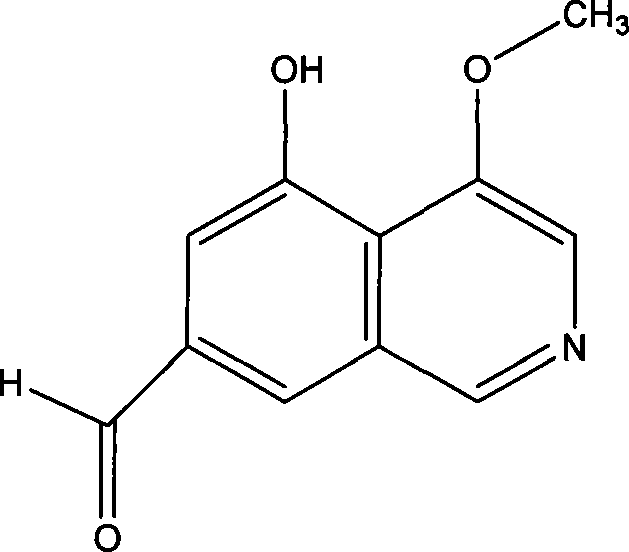
[0031] The structure identification of embodiment 2 compound (3-methoxy group-5 hydroxyl group-7-formyl isoquinoline)
[0032] The physical and chemical properties of the compound: the compound (3-methoxy-5-hydroxyl-7-formyl isoquinoline) is a yellow needle-like crystal (methanol), easily soluble in chloroform, ethyl acetate, acetone, soluble in methanol, hardly Dissolved in water. ESI-MS: 226 (M+Na) + . 1 H NMR (CDCl 3 , 600Hz) δ: 9.96(1H, s), 9.17(1H, s), 8.52(1H, s), 7.95(1H, d, J=3.0Hz), 7.13(1H, dd, J=3.0, 0.6Hz ), 4.00 (3H, s). 13 C NMR (CDCl 3 , 600Hz) δ: 185.1, 164.7, 149.7, 148.2, 148.1, 130.3, 125.1, 124.4, 115.0, 113.5, 52.5.
[0033] Table 1 shows the two-dimensional NMR spectrum data (detection 1 Heteronuclear multiple quantum coherence (HMQC) of H, detected 1 Heteronuclear multiple bond correlation (HMBC) of H (see Figure 1).
[0034] Table 1 Two-dimensional NMR spectrum data
[0035] 1 Hδ
HMQC
HMBC
9.96 (1H, s)
9.17 (1H,...
Embodiment 3
[0038] The distribution of the compound (3-methoxy-5-hydroxyl-7-formyl isoquinoline) in different organs of sea lacquer
[0039] Collect the roots, stems and leaves of sea paint, dry them in the shade, take 100g each, and use a pulverizer to crush the samples into particles with a particle size of 0.1-0.5cm. Extract with 95% ethanol for 48 hours, repeat 3 times, combine the extracts, concentrate under reduced pressure with a rotary evaporator to obtain an extract. Obtain the extractum of root, stem, leaf and weigh 7.35g, 5.04g, 3.84g respectively. The obtained extract is detected by TLC, and compared with the pure product of the compound obtained, the compound itself is yellow, and it can be seen from TLC that the extracts of leaves and stems do not have spots corresponding to the samples, while the extracts of roots have very obvious spots. spots. It was determined that the active substance is mainly distributed in the roots.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com