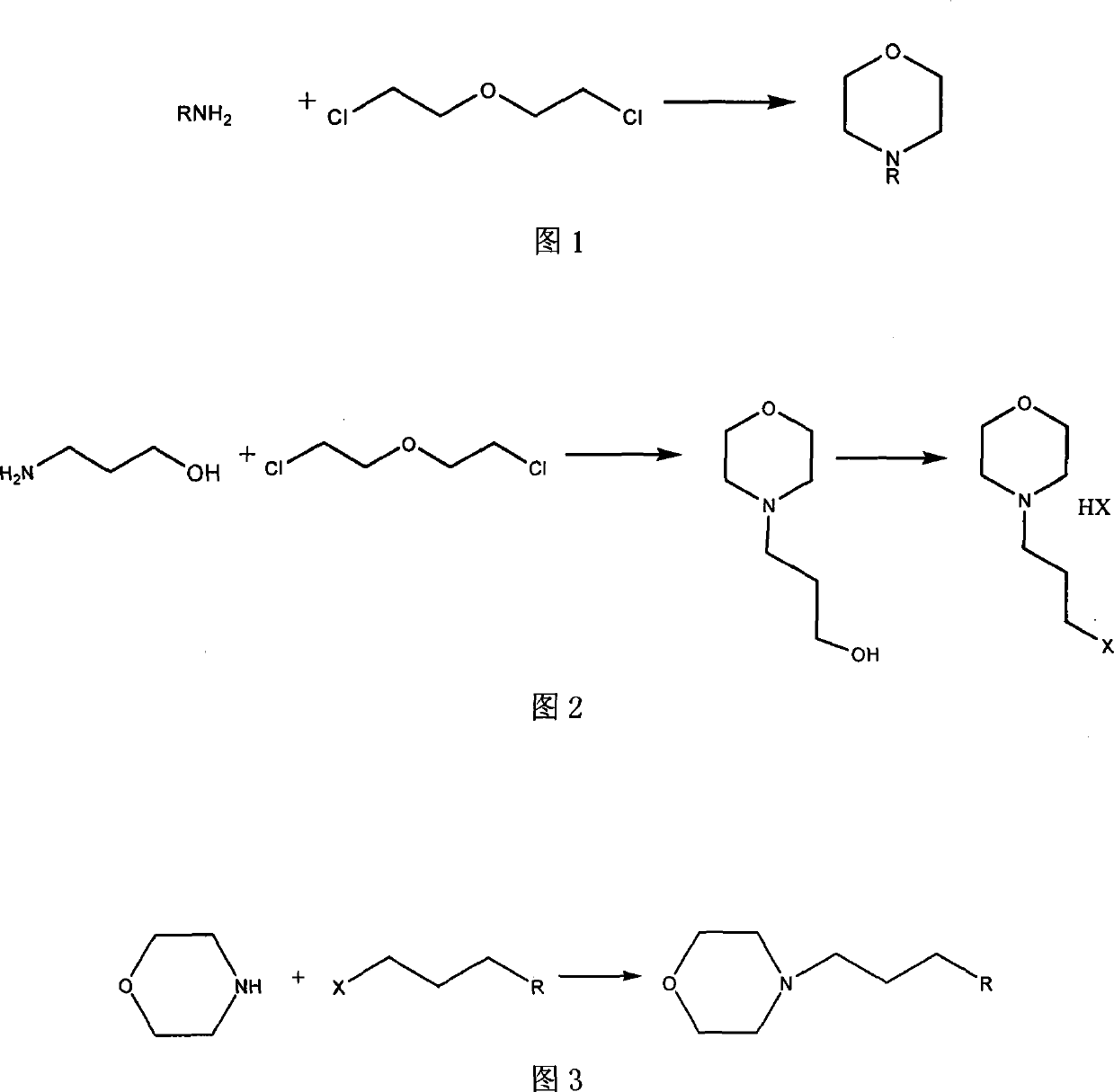
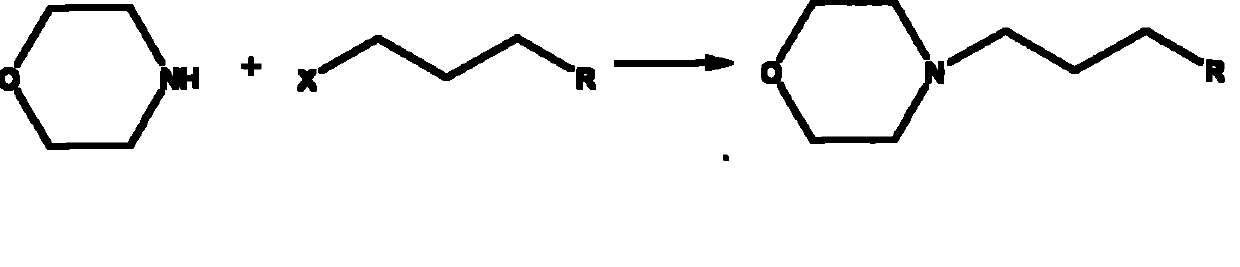
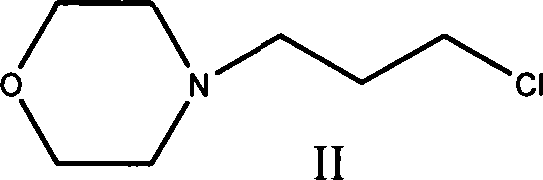
Technique for preparing N-substituted morpholines organic compounds
An organic compound and preparation process technology, applied in the direction of organic chemistry, etc., can solve the problems of high price of toluene as a solvent, low flash point of toluene, unsuitable for amplification, etc., and achieve obvious cost and technical advantages, low price and stable yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In a 250ml three-necked flask, add morpholine (10.5g, 0.12mol), sodium hydroxide (4.80g, 0.12mol), bromoethane (13.8g, 0.12mol), potassium iodide (1.0g, 6mmol), acetone (30ml ) and a stirrer, stirred and reacted under reflux at 56° C. for 5 hours. Stop the reaction and filter. The filter cake was washed with acetone, the filtrates were combined, and the acetone was evaporated. Add 60ml of dichloromethane, wash once with 20ml saturated sodium bicarbonate solution, and once with 20ml saturated brine. Dichloromethane was distilled off to obtain 14 g of a pale yellow liquid. It was distilled under reduced pressure, and the fraction at 68°C was collected to obtain a colorless liquid (8.4 g, yield 60.5%). 1H-NMR (TMS, CDCl3) δ 3.73 (t, J = 7.0Hz, 4H), 2.44 (t, J = 6.8Hz, 4H), 2.41 (m, 2H), 1.09 (t, 3H).
[0031] Product Molecular Formula:
[0032]
Embodiment 2
[0034] In a 250ml three-necked flask, add morpholine (10.5g, 0.12mol), sodium hydride (2.88g, 0.12mol), 1-chloro-3-bromopropane (18.9g, 0.12mol), sodium bromide (0.12g, 1.2mmol), chloroform (60ml) and a stirrer, stirred and reacted under reflux at 61°C for 12 hours. Stop the reaction and filter. The filter cake was washed with chloroform, the filtrates were combined, and the chloroform was distilled off. Add 60ml of dichloromethane, wash once with 20ml saturated sodium bicarbonate solution, and once with 20ml saturated brine. Dichloromethane was distilled off to obtain 22 g of a yellow liquid. Distilled under reduced pressure, collected fractions at 80°C to obtain light yellow liquid (12.3 g, yield 63.0%). 1H-NMR (TMS, CDCl 3 ) δ 3.70 (t, j = 6.5 Hz, 4H), 3.61 (t, J = 6.5 Hz, 2H), 2.47 (m, 6H), 1.95 (m, 2H). Product Molecular Formula:
[0035]
Embodiment 3
[0037] In a 250ml three-necked flask, add morpholine (10.5g, 0.12mol), potassium carbonate (16.6g, 0.12mmol), allyl bromide (14.5g, 0.12mol), potassium bromide (0.29g, 2.4mmol), Methyl tert-butyl ether (150ml) and a stir bar were stirred at 0°C for 8 hours. Stop the reaction, filter, wash the solid with methyl tert-butyl ether, evaporate the combined filtrate to remove methyl tert-butyl ether, add 60ml of dichloromethane, wash once with 20ml saturated sodium bicarbonate solution, and once with 20ml saturated brine. Dichloromethane was distilled off to obtain 17.7 g of a pale yellow liquid. Distilled under reduced pressure, collected fractions at 72°C to obtain light yellow liquid (11.3 g, yield 73.9%). 1H-NMR (TMS, CDCl 3 )δ5.83 (m, 1H), 5.18 (d, 1H), 5.15 (s, 1H), 3.72 (t, 4H), 2.99 (d, 2H) 2.45 (t, 4H) product molecular formula:
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com