Platinum aluminium oxide series catalyst for preparing propylene by propane dehydrogenation and preparation method of catalyst
A technology of propane dehydrogenation, platinum alumina, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the limitations of chromium catalyst use, short catalyst life, Toxic problems such as metal chromium, to achieve the effect of easy to enlarge production and industrialization, less carbon deposition, slow decline in activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
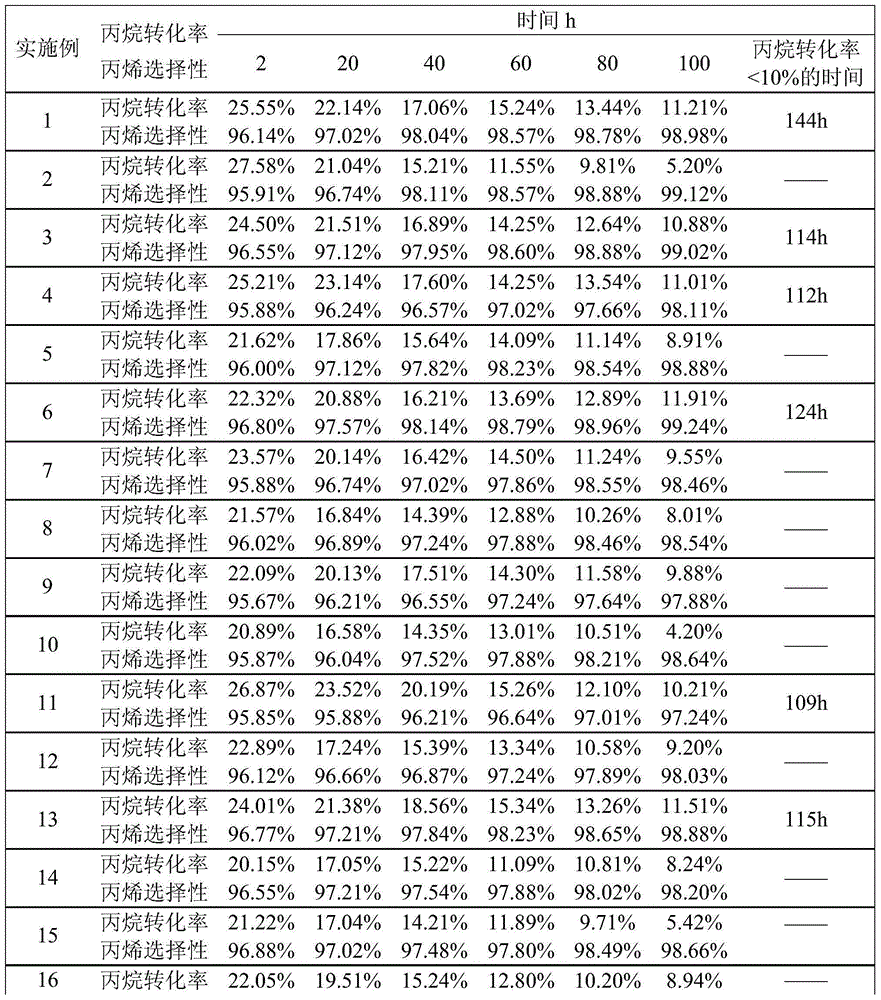
Embodiment 1
[0035]Platinum nitrate with a Pt content of 0.3 g, stannous chloride with a Sn content of 0.4 g, potassium nitrate with a K content of 0.6 g and cerium nitrate with a Ce content of 0.8 g were dissolved in 1% dilute nitric acid, and then 97.9 Pour g nano-alumina carrier into the above mixed solution, add an appropriate amount of 1% dilute nitric acid to ensure that the nano-alumina is completely immersed in the solution, then heat to 95°C, stir for 2h, filter and dry at 100°C for 6h, set it to The catalyst was obtained by roasting at 550℃ for 8h in a muffle furnace to obtain a catalyst for dehydrogenation of propane to propylene.
Embodiment 2
[0037] Dissolve platinum nitrate with a Pt content of 0.3 g in 2% dilute nitric acid, then pour 137.3 g of pseudo-boehmite into the above mixed solution, add an appropriate amount of 1% dilute nitric acid to ensure complete impregnation of pseudo-boehmite In the solution, it was heated to 88°C, stirred for 2h, filtered, then placed in an oven, dried at 100°C for 8h, and finally calcined at 550°C for 4h to obtain the catalyst precursor for dehydrogenation of propane to propylene.
[0038] According to the above method, Sn was impregnated on the catalyst precursor in the previous step. The source of Sn was stannous chloride, and the Sn content was 1.8 g. Finally, Na and Zn were impregnated on the catalyst precursor. Na and Zn were respectively obtained from nitric acid. The contents of sodium and zinc nitrate are 0.6 g and 1.2 g respectively, to obtain a catalyst for dehydrogenation of propane to propylene.
Embodiment 3
[0040] Dissolve barium nitrate and cerium nitrate containing 0.7 g and 1.3 g of Ba and Ce in 1% dilute nitric acid, then pour 97.0 g of alumina microspheres into the above mixed solution, add an appropriate amount of 2% dilute nitric acid to Make sure that the alumina microspheres are completely immersed in the solution, then heated to 95°C, stirred for 3h, filtered, placed in an oven, dried at 100°C for 6h, and finally calcined at 550°C for 5h to obtain the catalyst precursor for propane dehydrogenation to propylene.
[0041] According to the above method, Sn was impregnated on the catalyst precursor in the previous step. The Sn source was stannous chloride, and the Sn content was 0.7 g. Finally, Pt was impregnated on the catalyst precursor. Pt came from platinum nitrate, and its Pt content was It is 0.3g, and the catalyst for dehydrogenation of propane to propylene is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com