Application of sterides in preparing medicament for treating cardiovascular and cerebrovascular diseases
A technology for cardiovascular and cerebrovascular diseases and steroidal compounds, which is applied in the application field of steroidal compounds in the preparation of drugs for treating cardiovascular and cerebrovascular diseases. , resource-rich effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
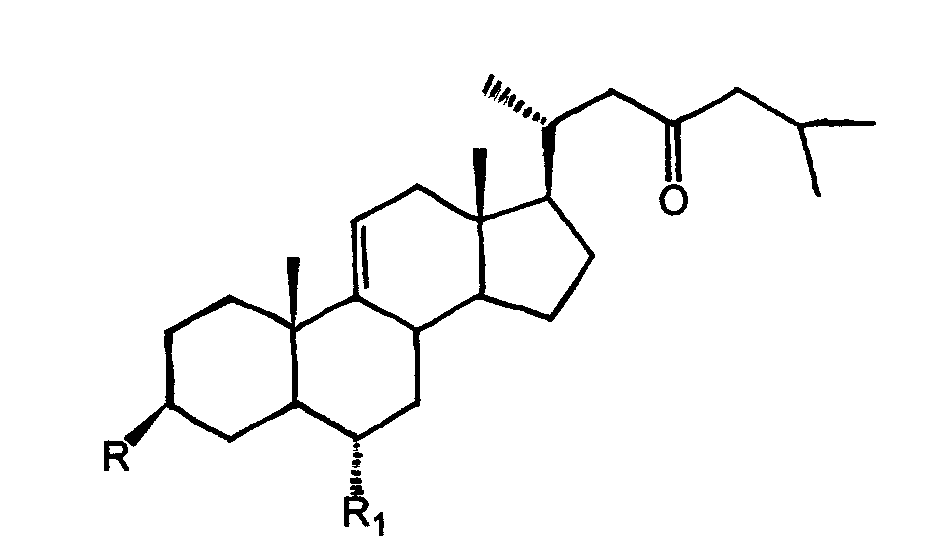
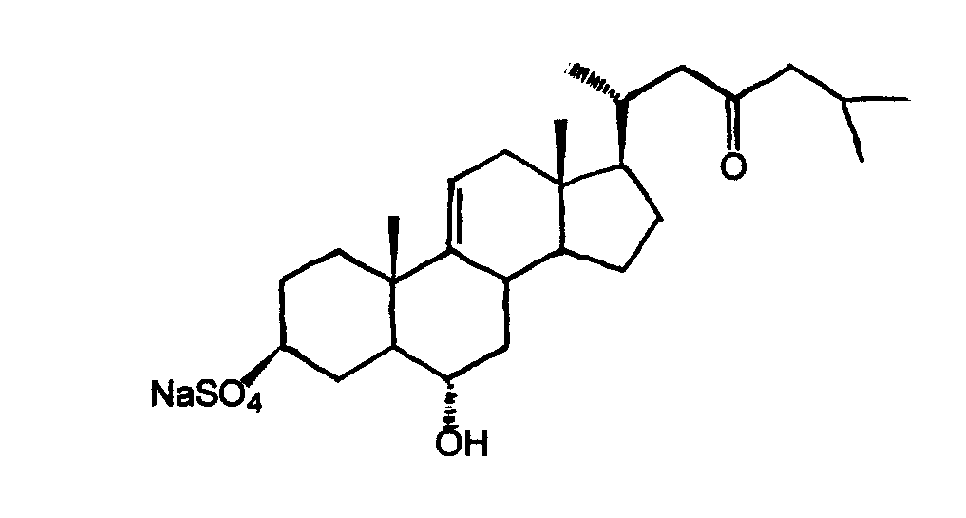
[0016] Embodiment 1 steroid compound and its preparation
[0017] Asterias amurensis (Asterias amurensis) 4 kg, methanol reflux extraction 3 times (24L×3), methanol recovery under reduced pressure. The methanol extract was dispersed in 4 L of water, extracted three times with an equal volume of n-butanol, combined, and concentrated under reduced pressure to obtain 350 g of n-butanol extract. Get the above-mentioned n-butanol extract, through silica gel column chromatography (Φ30×80cm), chloroform methanol mixed solution (chloroform and methanol according to volume ratio 100:0, 50:1, 20:1, 10:1, 8:2, 7:3, 6:4 ratio) gradient elution, every 1000 milliliters was collected as a fraction, and then combined according to the results of thin layer chromatography to obtain 15 fractions (fraction A to O); fraction H (chloroform-methanol 8: 2 elution fractions) 20 grams of ODS medium and low pressure column chromatography, methanol-water (volume ratio 30%, 50%, 70%, 90%) gradient elutio...
Embodiment 2
[0023] Embodiment 2 promotes the detection of vasodilation activity
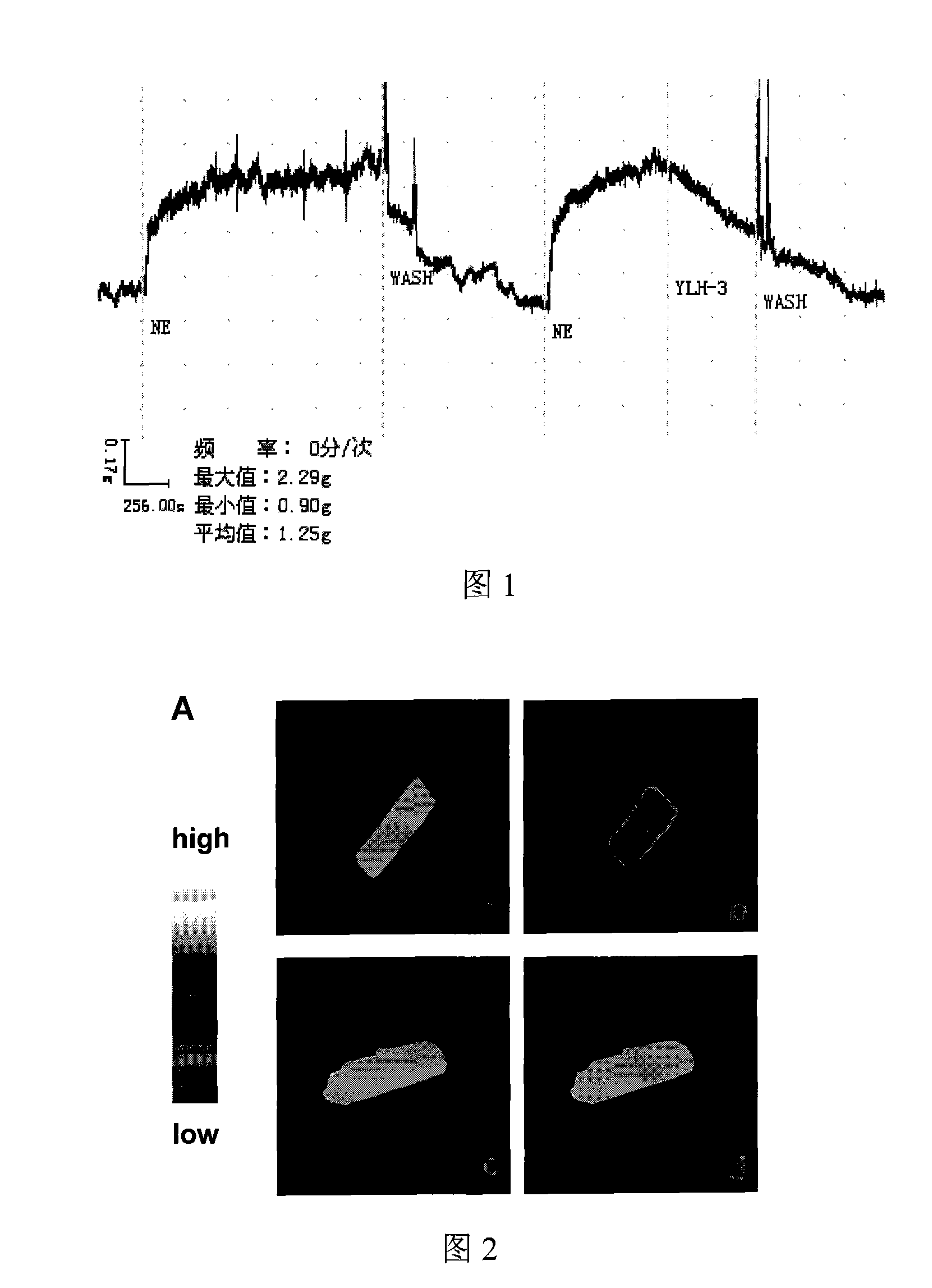
[0024] Accurately weigh the steroid compound obtained in Example 1, add DMSO, shake and dissolve to form 1mol L -1 solution. Take the rat thoracic aorta, cut it into a 3mm wide arterial ring, then hang the arterial ring in a bath filled with normal saline, with a volume of 20ml, and continuously pass it with 95% O 2 +5%CO 2 of mixed gas. One end of the arterial ring was fixed, and the other end was connected to the tension sensor of the BL-420 biological signal recording system with a thin wire, and a preload of 1 gram was given. Add norepinephrine after one hour of stabilization. After elution, stabilize for 20 minutes, add norepinephrine, add 10 μM steroid compound (coded as YLH-3) obtained in Example 1 at the peak of norepinephrine contraction, and record the tension curve. The tension curve against the effect of norepinephrine-induced vasoconstriction is shown in FIG. 1 .
[0025] It can be seen fr...
Embodiment 3
[0026] Example 3 Protective Effects on Calcium Overload in Cardiomyocytes Induced by KCl
[0027] Accurately weigh the steroid compound obtained in Example 1, add DMSO, shake and dissolve to form 1mol L -1 solution. Single rat ventricular myocytes were separated by enzymatic hydrolysis, and the cardiomyocytes were placed in 20 μmol·L -1 In the loading solution of Fluo-3 / AM, after Fluo-3 / AM passively diffuses into the cells, it is hydrolyzed by esterase to release Fluo-3, Fluo-3 and Ca 2+ Complexation, excited by 488nm laser to generate fluorescence, its fluorescence value is the same as [Ca 2+ ] i It is positively correlated, that is, it is expressed by the change of fluorescence value [Ca 2+ ] i Changes, the XY plane of the cell was scanned under the timeseries program of the laser scanning confocal microscope with an interval of 10s, and a total of 25 scans were performed. Calculate the average fluorescence intensity in the XY plane of the cell, and use the average flu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com