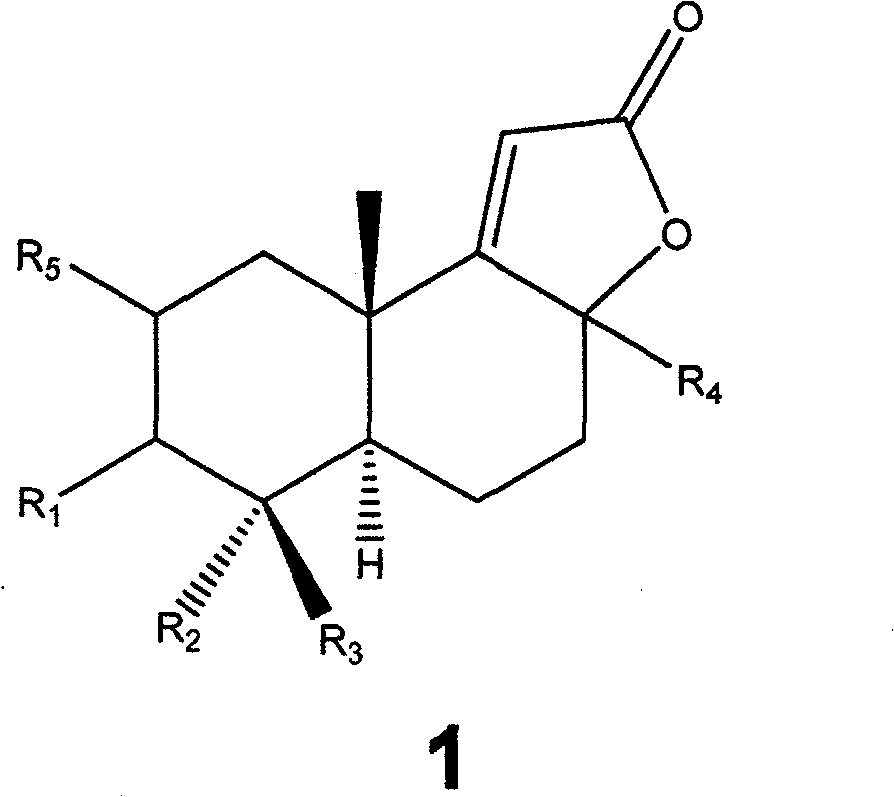
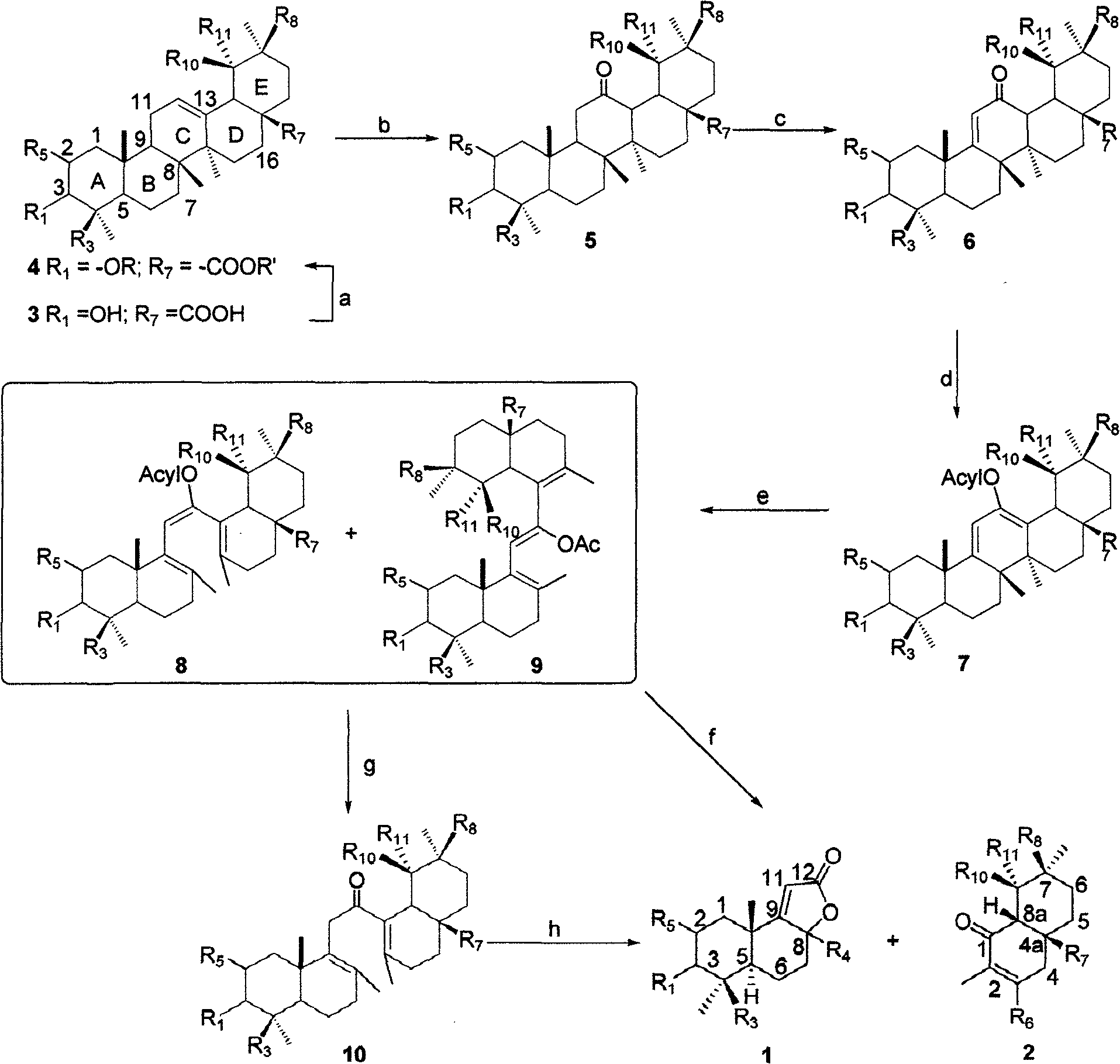
Polysubstitution hydrogenated naphthalene compounds, producing method and uses of the same
A compound, hydrogenated naphthalene technology, applied in the direction of organic chemistry, etc., can solve the problem of quality can not be guaranteed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Using oleanolic acid as raw material to synthesize compounds 1 and 2
[0046] 1) Preparation of Compound 4:
[0047]
[0048] Dissolve 5 g of compound 3 in 20 mL of dry chloroform, add 10 mL of anhydrous methanol and 1 mL of concentrated sulfuric acid, and then heat it to slowly reflux for 6 h. After cooling, the reaction mixture was successively washed with water, saturated sodium bicarbonate and saturated brine, and dried over anhydrous magnesium sulfate. The desiccant was filtered off and distilled under reduced pressure, and the obtained solid was dried in vacuo. Then it was dissolved in 20 mL of dry chloroform, 5 mL of dry pyridine and 3 mL of acetic anhydride were added, and stirred at room temperature for 12 h. The reaction mixture was washed successively with water, saturated aqueous sodium bicarbonate solution, water and saturated brine. After drying over anhydrous magnesium sulfate and distilling under reduced pressure, a white solid was obtain...
Embodiment 2
[0073] Example 2 Using maslinic acid as raw material to synthesize compounds 1 and 2
[0074] Using the operation steps described in implementation 1, using maslinic acid as raw material, the compounds 1 and 2 obtained are as follows:
[0075]
Embodiment 3
[0076] Example 3 Using ursolic acid as raw material to synthesize compounds 1 and 2
[0077] Adopt as implementing the described operating steps of 1, take ursolic acid as raw material, the compound 1 and 2 that obtain are as follows:
[0078]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com