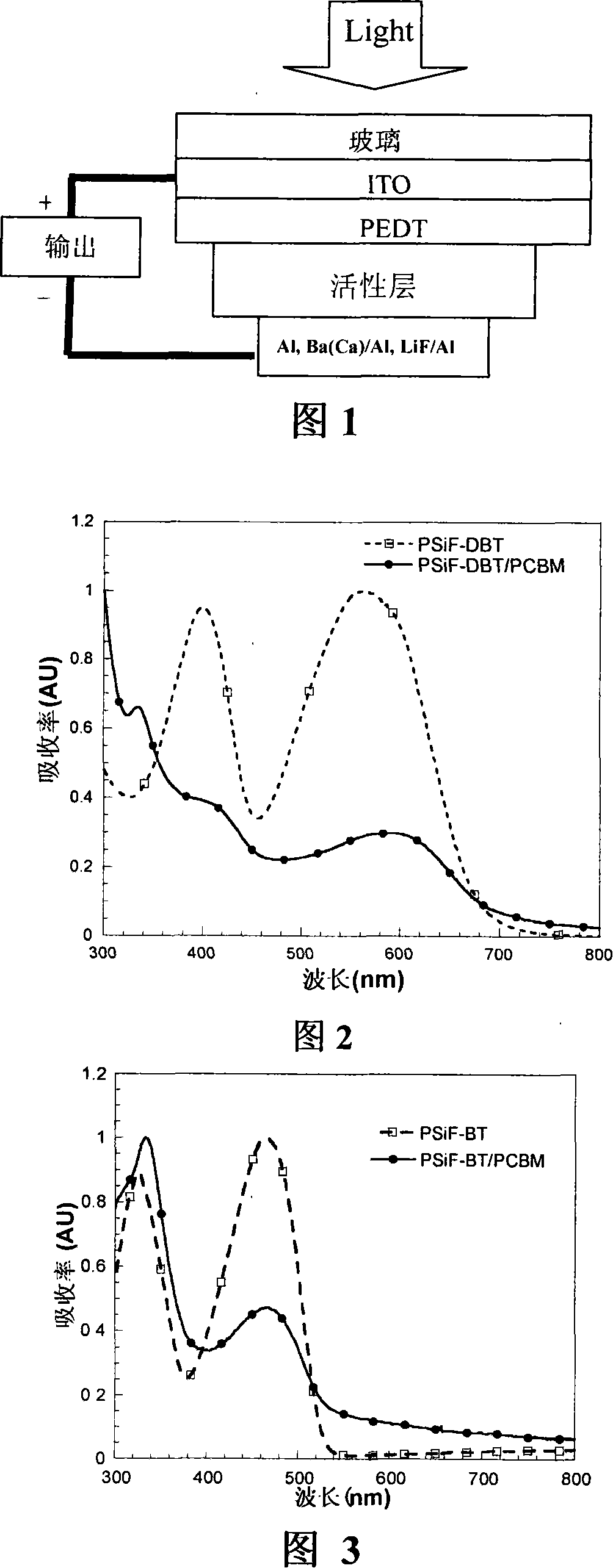
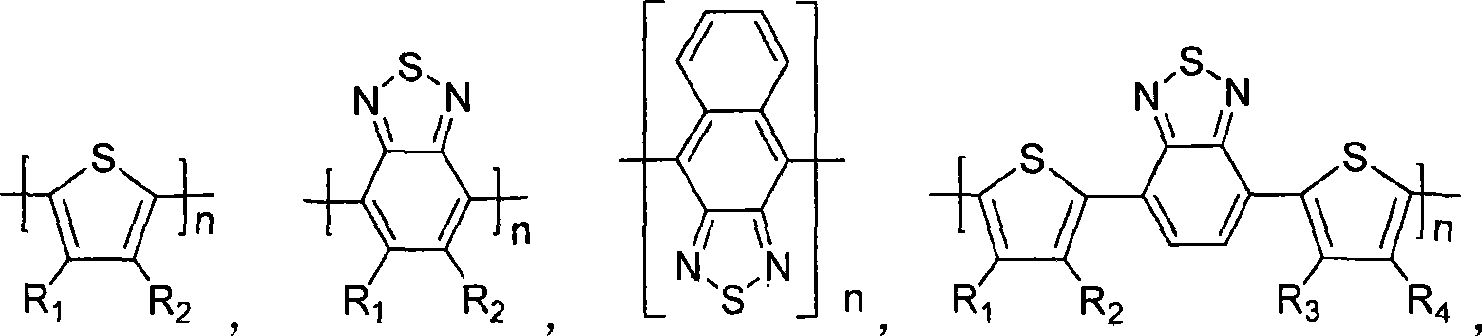
Silicon-containing fluorine conjugated polymer and its preparing process and application
A conjugated polymer, polymer technology, used in final product manufacturing, sustainable manufacturing/processing, semiconductor/solid-state device manufacturing, etc., can solve problems such as inapplicability, reduce electronegativity, and reduce the possibility of oxidation good photothermal oxidative stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of 2,7-dibromo-9,9-disubstituted silfluorene, the synthetic route is as follows:
[0037]
[0038] According to the method disclosed in J.Am.Chem.Soc.2005, 127, 7662, the preparation of 2,7-dibromo-9,9-dioctylsilafluorene is taken as an example to illustrate.
[0039] (1) 4,4'-dibromo-2,2'-dinitrobiphenyl
[0040] Add 50g (0.178mol) of 2,5-dibromonitrobenzene and 45g (0.70mol) of copper powder into a 500mL one-necked flask, and then add 200mL of N,N-dimethylformamide. Heated to 120°C and reacted for 5 hours. After cooling to room temperature, 100 mL of toluene was added. The residue was filtered off, and the filtrate was washed with salt and water. After spin-dried, recrystallize with a mixed solvent of methanol:toluene=4:1. Finally, pale yellow crystals were obtained.
[0041] (2) 4,4'-dibromo-2,2'-diaminobiphenyl
[0042] Add 4,4'-dibromo-2,2'-dinitrobiphenyl (7.5g, 18.6mmol), 55mL of hydrochloric acid, tin powder (9g, 75.8mmol) and 90mL...
Embodiment 2
[0047] Example 2 Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-9,9-disubstituted silfluorene
[0048] According to the method disclosed in J.Am.Chem.Soc.2005, 127, 7662, the preparation of 2,7-diboronate-9,9-di-n-octylsilafluorene is taken as an example to illustrate.
[0049] Under nitrogen protection, 2,7-dibromo-9,9-dioctylsilafluorene (4.45g, 7.9mmol) was dissolved in 80mL THF and cooled to -78°C, and n-butyl lithium (12.4 mL, 1.6M n-hexane solution). After dripping, react at -78°C for another 2 hours, then add 2-isopropyl-4,4,5,5-tetramethyl-1,3,2-dioxaborane (8mL, 20mmol), Naturally raised to room temperature and stirred overnight. The reaction mixture was poured into water and extracted with ether. The organic layer was washed with water, spin-dried and passed through a column with silica gel. (petroleum ether: ethyl acetate 20:1). It was further purified by recrystallization from methanol. Finally a white solid was obtained. (4.0g, 77%)
...
Embodiment 3
[0052] Example 3 Preparation of 4,7-dibromo-2,1,3-benzothiadiazole, the molecular structure of which is shown below.
[0053] 4,7-Dibromo-2,1,3-benzothiadiazole was prepared according to the method disclosed in J.Heterocycle.Chem., 1970, 7,629. Weigh benzothiadiazole (13.6 g, 0.1 mol), dissolve it in 20 ml of 45% hydrobromic acid, heat and stir until reflux, and add bromine (48 g, 0.3 mol) dropwise. After the dropwise addition was completed, an additional 10 ml of hydrobromic acid was added and reflux was continued for 2.5 hours. The reaction mixture was filtered while it was hot, cooled, filtered again, washed with water, dried, and recrystallized from chloroform to obtain 4,7-dibromo-2,1,3-benzothiadiazole.
[0054]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com