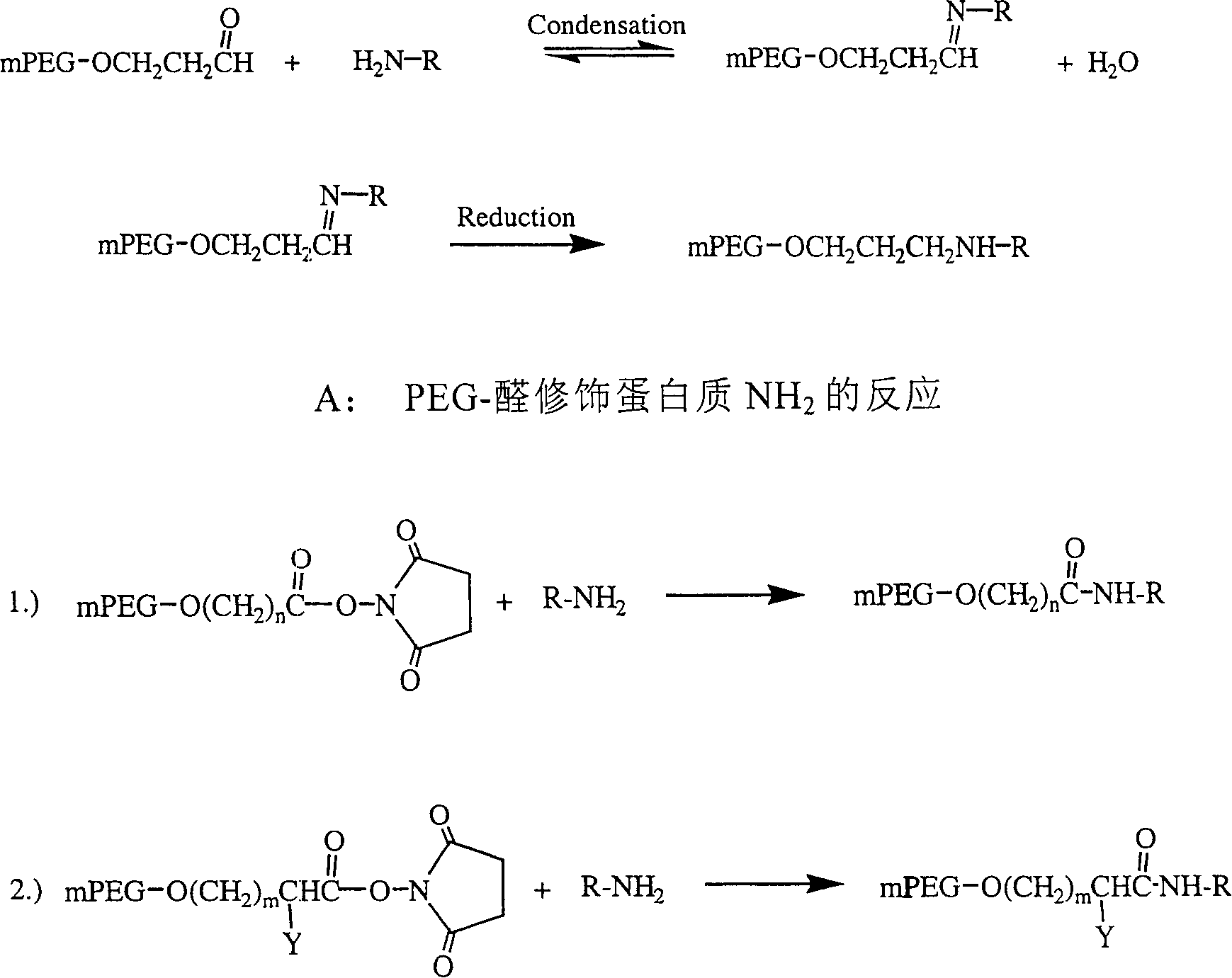
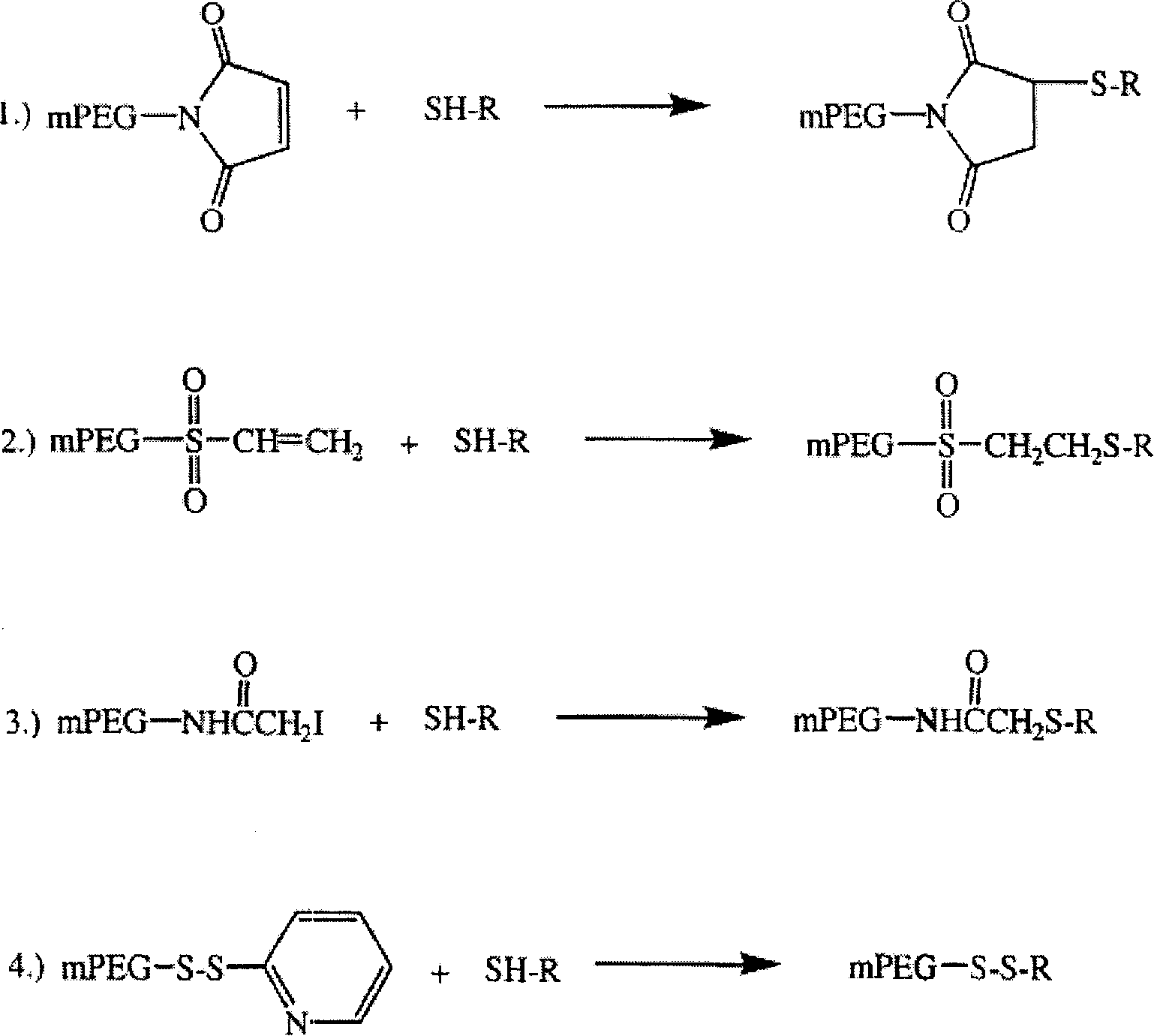
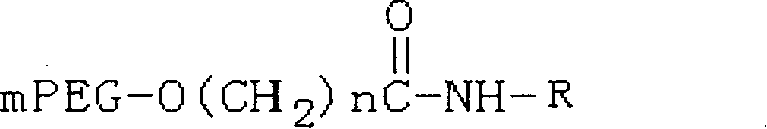Polyethylene decorative nevin fibrinolytic enzyme and its preparation method
A technology of polyethylene glycol and plasmin, which is used in biochemical equipment and methods, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of poor stability and short half-life, and achieve Good stability, low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Snake venom fibrinolytic enzyme modified by methoxypolyethylene glycol succinimidyl propionate 5000 (mPEG-SPA-5000)
[0042] The choice of reaction temperature: get 1.0mg / ml fibrinolytic enzyme solution (from the fibrinolytic enzyme of Agkistrodon venom of Changbai Mountain, hereinafter the same) 2ml, add 2ml phosphate buffer saline to make the pH value of solution be 7.6, then add mPEG- Dissolve 2.0 mg of SPA-5000 solid, mix well, take 0.3 ml each and place in 4 test tubes with stoppers, then place them at 4°C, 10°C, 25°C and 37°C for 30 minutes to stop the reaction. Compare the modification rate and determine the modification condition. The results showed that the polyethylene glycol-modified plasmin could be obtained at these temperatures, and the modification rate was the highest at 25°C. See Table 1 for specific data.
[0043] Table 1: Effects of different temperatures on the modification rate of plasmin
[0044] temperature
4℃
10℃
25℃
...
Embodiment 2
[0052] Isolation, Purification and Identification of Polyethylene Glycol Modified Plasmin
[0053] Take 2ml of 1.0mg / ml plasmin solution, add about 5ml of phosphate buffer to make the pH of the solution 7.6, then add 2.0mg of mPEG-SPA-5000 solid, dissolve, mix well, and react at 25°C for 30min. The reaction was terminated by adding 3 g of glycine solid.
[0054] The above reaction solution was taken, dialyzed against 0.05 mol / L Tris-HCl buffer solution with pH 7.8, concentrated to 5 ml with an ultrafiltration membrane with a molecular weight cut-off of 10,000, and separated on a column. The chromatographic conditions are as follows:
[0055] Chromatography medium: SOURCE 30Q
[0056] Column volume: 5ml
[0057] Flow rate: 1.0ml / min
[0058] Column equilibration: equilibrate 5 times column volume with 0.05mol / L, pH 7.8 Tris-HCl (starting buffer)
[0059] Sample volume: 5ml
[0060] Elution: First use 3 times the column volume of starting buffer to elute the unadsorbed par...
Embodiment 3
[0073] Determination of the biological potency (specific activity) retention rate of the modified product (see the State Food and Drug Administration for the method: National Drug Standard: Plasmin)
[0074] According to the titer determination method of plasmin in drug standard, measure the titer of plasmin and the plasmin modified with polyethylene glycol, then calculate the specific activity (U / mg) of sample. The results are shown in Table 4.
[0075] Table 4 Defibrase and the biological potency of the defibrase modified with polyethylene glycol
[0076] Sample specific activity (U / mg)
[0077] Plasmin 1500
[0078] PEG-modified plasmin 800
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com