Oral solid dosage forms containing a low dose of estradiol
A solid dosage form, estradiol technology, applied in the field of oral solid dosage forms, can solve problems such as being unsuitable for oral administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
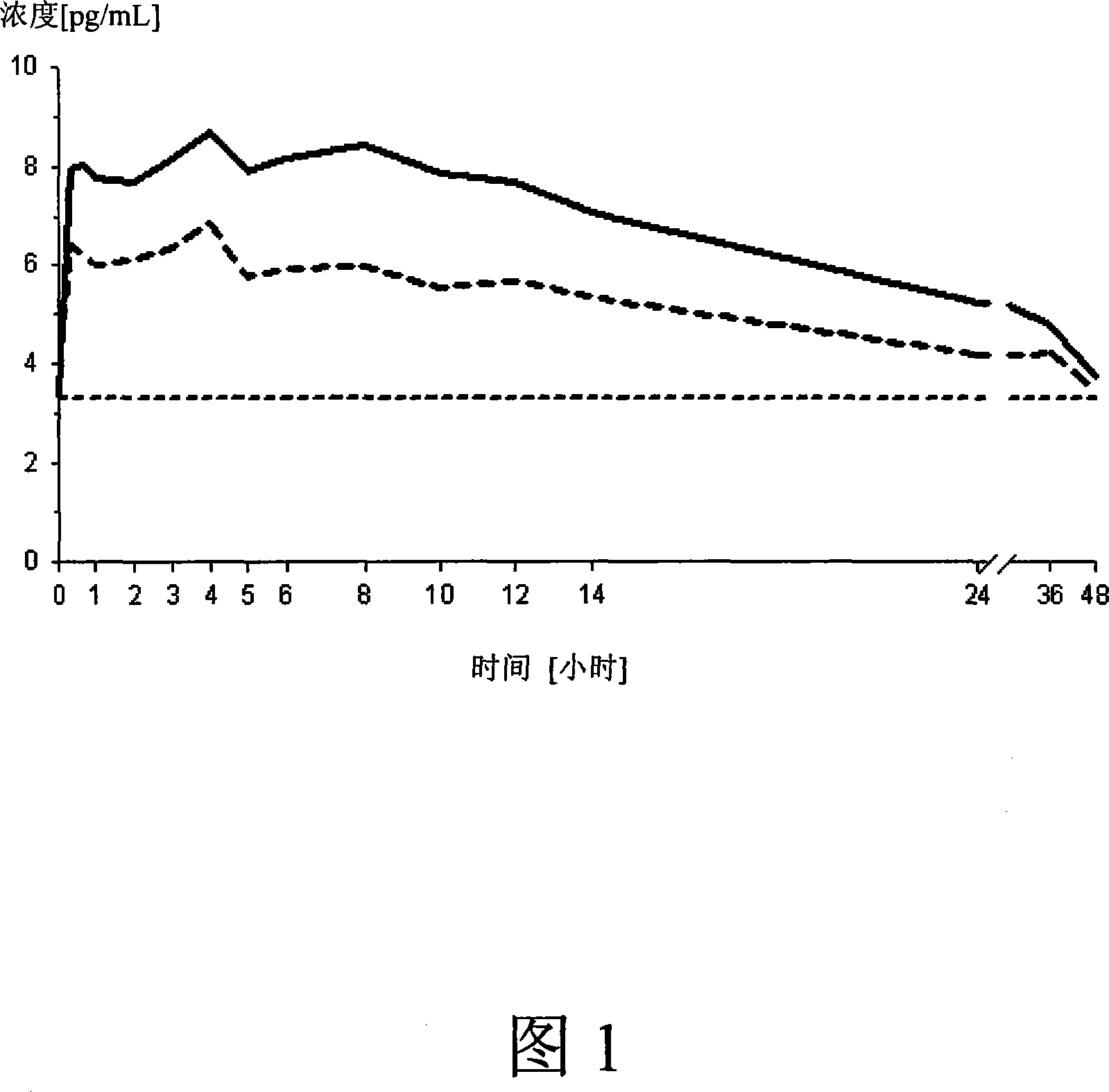
[0103] Example 1 - In Vitro Dissolution of Micronized Estradiol
[0104] The dissolution profiles of micronized estradiol and mixtures of micronized estradiol and polyvinylpyrrolidone were tested in an in vitro dissolution test system. The dissolution tester uses USP apparatus 2 (paddle system) (see USP 27), using 900ml of purified water at a temperature of 37°C as the dissolution medium, and the rotation speed is 50rpm. Estradiol and mixture in powder form in an amount equivalent to 100 μg of micronized estradiol hemihydrate were placed in the dissolution vessel.
[0105] Table 1. Dissolution test results
[0106] powder ingredients
following time
Dissolution amount of estradiol (μg):
5min
10min
20min
30min
60min
100 μg estradiol
100μg estradiol + 100μg
100μg Estradiol + 4mg
<3 *
16
19
<3 *
29
47
<3 *
48 ...
Embodiment 2
[0109] Example 2- Preparation of Oral Solid Dosage Form of Low Dose Estradiol
[0110] Prepare 80 mg tablet cores with the following composition:
[0111] Element:
Quantity (mg):
I Estradiol (hemihydrate, micronized)
II lactose monohydrate
III corn starch
IV modified starch (corn)
0.05
not higher than 80mg
14.40
9.60
2
0.80
0.1
not higher than 80mg
14.40
9.60
2
0.80
0.19
not higher than 80mg
14.40
9.60
2
0.80
[0112] The corn starch marked "V" may be replaced with another "second choice" binder, eg (low substituted) hydroxypropyl cellulose in an amount of 1.6 mg.
[0113] Oral solid dosage forms are prepared by adding ingredients I-IV (estradiol, lactose monohydrate, corn starch, and modified starch) to a fluid bed granulator and starting the fluid bed. The aqueous solution of ingredient V (corn starch) was t...
Embodiment 3
[0114] Example 3 - Stability of low dose estradiol dosage forms
[0115] The chemical stability of estradiol (semi-hydrated and micronized) in three different tablet formulations A, B and C was tested in the dark at 40°C and 75% relative humidity for 12 months. Keep tablets in an airtight container during storage.
[0116] At the start of the stability study, the three tablet formulations (A, B, and C) contained a labeled value of 0.05 mg of micronized estradiol hemihydrate and were supplied with hydroxypropylcellulose, polyethylene glycol , talc, titanium dioxide and iron oxide pigment yellow coating film. The 80 mg tablet core has the following composition ("+" indicates the presence of a given component):
[0117] Element
Quantity (mg)
A
B
C
I Estradiol (hemihydrate, micronized)
II lactose monohydrate
III corn starch
IV modified starch (corn)
V corn starch
VI magnesi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com