Preparation method of alpha-aza toroid drug template
A technology for azaspirocycles and drugs, which is applied in the field of preparation of spirocyclic drug templates, can solve the problems of low yield and many reaction steps in the synthesis method, and achieve the effects of high yield, easy industrial operation, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
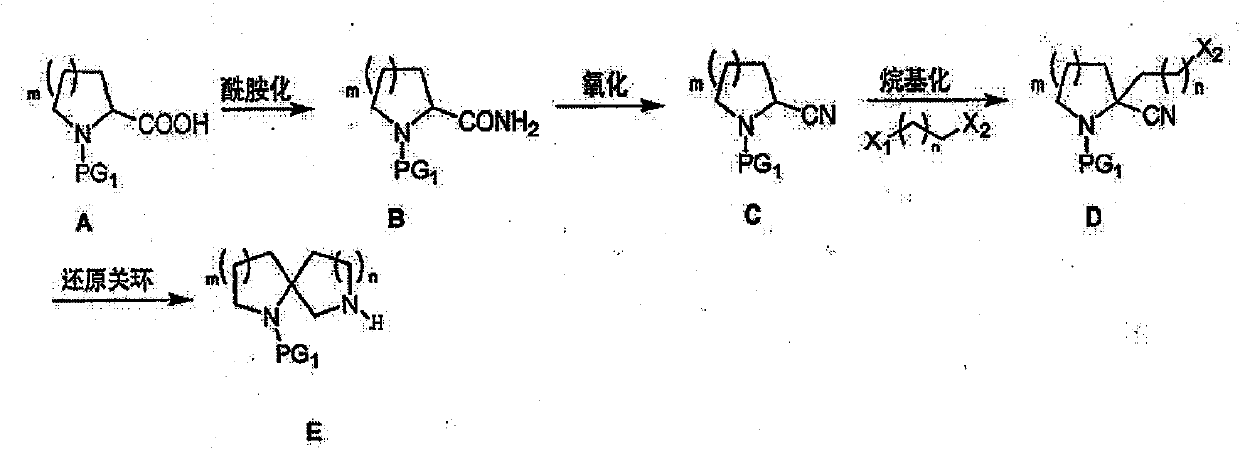
[0042]
[0043] 1. Synthesis of tert-butyl 2-formyl-pyrrole-1-carboxylate
[0044] Dissolve pyrrole-1,2-dicarboxylic acid-1-tert-butyl ester (10.75g, 0.05mol) and triethylamine (22.5g, 0.225mol) in dichloromethane (50ml), cool to -30°C and add chlorine Ethyl formate (10.8 g, 0.1 mol), NH was added after 30 min 3 .H 2 O (11 ml, 0.15 mol). The system was raised to room temperature and reacted overnight. The reaction system was washed with water, and the organic phase was washed with Na 2 SO 4 Drying and solvent removal gave tert-butyl 2-formyl-pyrrole-1-carboxylate (6.4 g, 60%).
[0045] 1 H-NMR (400MHz, CDCl 3 ): 5.50-7.01(m, 2H), 4.87(m, 1H), 4.35(m, 1H), 3.44(m, 2H), 1.75-1.99(m, 4H), 1.43(s, 9H); MS( m / z): 215 (M+1)
[0046] 2. Synthesis of tert-butyl 2-cyano-pyrrole-1-carboxylate
[0047] 2-Formyl-pyrrole-1-carboxylic acid tert-butyl ester (10.7 g, 0.05 mol) was dissolved in CH 2 Cl 2 (300ml) and Et 3 N (30.3g, 0.3mol), trifluoroacetic anhydride (20.1g, 0.1m...
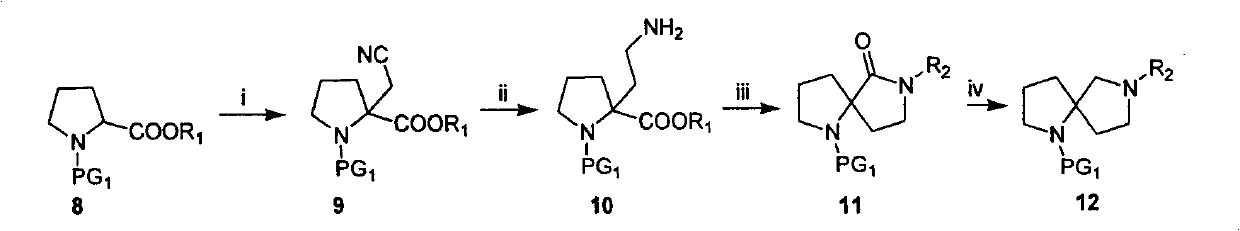
Embodiment 2
[0056]
[0057] Refer to Example 1 for the synthesis of tert-butyl 2-cyano-pyrrole-1-carboxylate.
[0058] 1. Synthesis of tert-butyl 2-(3-chloro-propyl)-2-cyano-pyrrole-1-carboxylate
[0059] At -78°C, a THF (30ml) solution of 2-cyano-pyrrole-1-carboxylic acid tert-butyl ester (4.9g, 0.025mol) was added to a THF solution of LDA (0.0375mol), and the reaction was continued for 45min. At -78°C, 1,3-dichloropropane (2.8 g, 0.025 mol) was added, the system was raised to room temperature, and reacted overnight. NH 4 The Cl solution was quenched, extracted with EtOAc, and the organic phase was dried and stripped to obtain the crude product tert-butyl 2-(3-chloro-propyl)-2-cyano-pyrrole-1-carboxylate (6.9 g). The product was subjected to column chromatography to obtain tert-butyl 2-(3-chloro-propyl)-2-cyano-pyrrole-1-carboxylate (5.1 g, 75%).
[0060] 1 H-NMR (400MHz, CDCl 3 ): 3.31-3.75(m, 4H), 2.35-2.51(m, 2H), 1.70-2.25(m, 6H), 1.51(s, 9H); MS(m / z): 295(M+23)
[0061] 2.1...
Embodiment 3
[0065]
[0066] 1. Synthesis of tert-butyl 2-formyl-piperidine-1-carboxylate
[0067] Dissolve 1-tert-butyl piperidine-1,2-dicarboxylate (11.45g, 0.05mol) and triethylamine (40.4g, 0.4mol) in dichloromethane (50ml), cool to -20°C and add chlorine Isopropyl formate (6.1 g, 0.05 mol), NH was added after 30 min 3 .H 2 O (18ml, 0.25mol). The system was raised to room temperature and reacted overnight. The reaction system was washed with water, and the organic phase was washed with Na 2 SO 4 Drying and solvent removal gave tert-butyl 2-formyl-piperidine-1-carboxylate (6.8 g, 60%).
[0068] 1 H-NMR (400MHz, CDCl 3 ): 6.12(m, 2H), 4.70(m, 1H), 4.11(m, 1H), 2.70(m, 1H), 2.25(m, 1H), 1.46(s, 9H), 1.38-1.70(m, 5H); MS (m / z): 229 (M+1)
[0069] 2. Synthesis of tert-butyl 2-cyano-piperidine-1-carboxylate
[0070] tert-butyl 2-formyl-piperidine-1-carboxylate (11.4 g, 0.05 mol) was dissolved in CH 2 Cl 2 (300ml) and Et 3 N (25.3g, 0.25mol), trifluoroacetic anhydride (50.0g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com