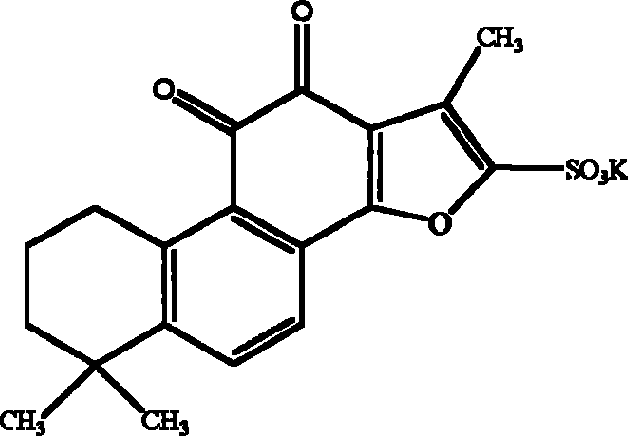
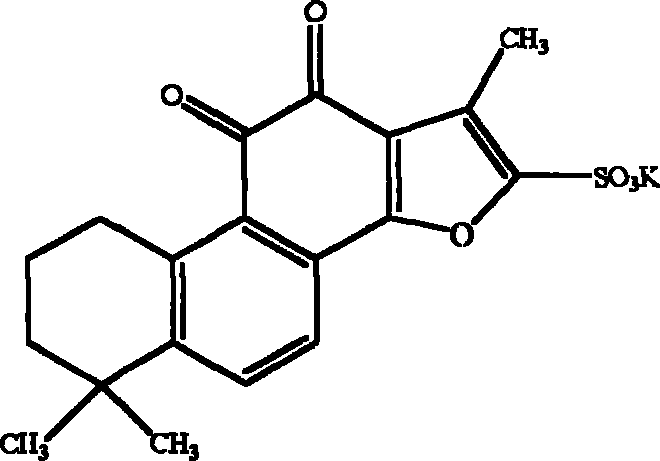
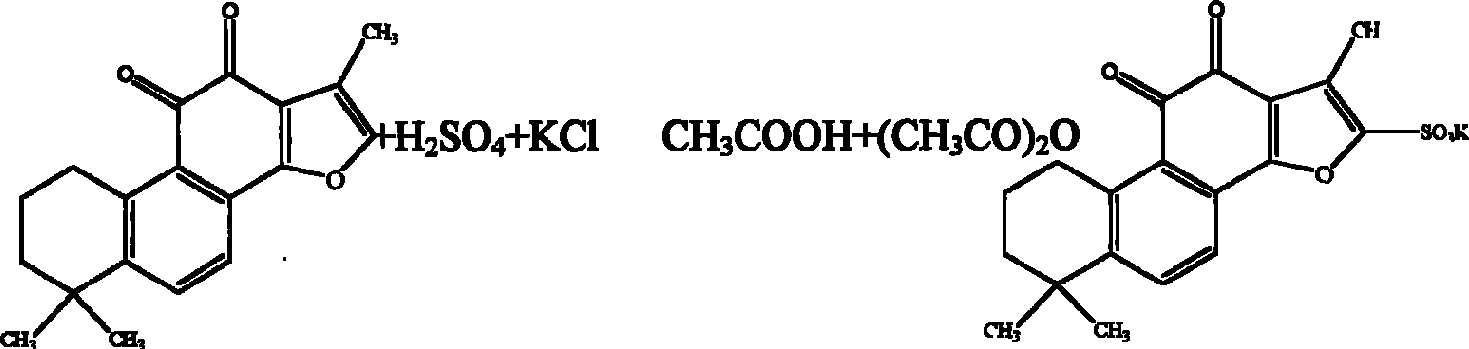Tanshinone IIA potassium sulfonate used for preparing medicine for preventing and treating cerebral ischemia and cerebral anoxia and myocardial ischemia and myocardio anoxia
A technology of tanshinone and potassium sulfonate, applied in the field of preparation of medicaments for preventing and treating myocardial ischemia hypoxia and cerebral ischemia hypoxia by tanshinone IIA potassium sulfonate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Extraction, separation and purification of Tanshinone IIA in Salvia miltiorrhiza
[0042]Dried Salvia miltiorrhiza 1kg, extracted with 95% ethanol for 3 times, each time for 24 hours, the extract was concentrated under reduced pressure to 500mL, then added 500mL of water, extracted four times with 1000mL chloroform, the extract was concentrated under reduced pressure, and the column layer Analysis and separation, the silica gel is 100-200 mesh, the eluent is petroleum ether / ethyl acetate mixed solution containing 1%-10% ethyl acetate, and gradient elution is carried out. About 0.85g of tanshinone IIA can be obtained.
Embodiment 2
[0043] The synthesis of embodiment 2 Tanshinone IIA potassium sulfonate
[0044] The synthesis process of potassium tanshinone IIA sulfonate: take 50 grams of tanshinone IIA (purity above 20%), add 200 milliliters of acetic anhydride, place it in a three-necked bottle, add 100 milliliters of concentrated sulfuric acid dropwise at 10-15 °C under stirring- The mixed solution of glacial acetic acid (1:1, V / V), after the dropwise addition, was stirred at room temperature for one hour, then the reaction solution was slowly poured into an equal volume of distilled water, and 1 liter of saturated potassium chloride ( Chemically pure) aqueous solution, that is, crude potassium tanshinone IIA sulfonate is precipitated, centrifuged, the precipitate is washed twice with saturated potassium chloride solution, and then washed once with an appropriate amount of water to make the pH of the solid matter = 5.6, and the filtered solid matter is placed on a water bath Evaporate to dryness, reflu...
Embodiment 3
[0045] Embodiment 3: Toxicological test of potassium tanshinone IIA sulfonate
[0046] Acute toxicity test in mice: The animals used in the test were healthy male Kunming mice. Tested by intravenous method. The dosage is 100mg / kg (the maximum amount has been reached). After the experiment, the mice were fed and observed for 7 days under the condition of free drinking and eating at 20±1°C, and the survival and appearance of the mice were observed.
[0047] Results: After administration, all the mice survived, and the appearance and behavior of the survivors were normal, and LD could not be detected. 50 . It is determined that potassium tanshinone IIA sulfonate has no toxic and side effects within the range of effective therapeutic dose and can be taken for a long time.
[0048] 30-day toxicity test on mice: The animals used in the test were healthy male Kunming mice. Tested by intravenous method. The dosage is 50mg / kg / day for 30 consecutive days. The test showed that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com