A kind of antioxidant peptide derived from hickory chick and its preparation method and application
An anti-oxidative peptide, morel technology, applied in the preparation method of peptide, chemical instruments and methods, application, etc., can solve the problem of lack of neuroprotective agents, and achieve the effect of slowing down the damage of oxidative stress on the nervous system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] An antioxidant peptide derived from morel, the sequence of the antioxidant peptide is:
[0051] Ala-Tyr-Asp-Gln-Ile-Asp-Ala-Ala-Pro-Glu-Glu-Lys.
[0052] The antioxidant peptide consists of 12 amino acids with a relative molecular mass of 1348Da.
Embodiment 2
[0054] Isolation, purification and sequence identification of antioxidant peptides derived from morel:
[0055] Weigh 100 g of Morchella importuna dry fruiting body, pulverize with a traditional Chinese medicinal material grinder, and pass through a 40-mesh sieve to obtain Morchella importuna dry fruiting body powder.
[0056] Configure phosphate buffered saline buffer (PBS buffer): Potassium dihydrogen phosphate (KH 2 PO 4 ) 0.27g, disodium hydrogen phosphate (Na 2 HPO 4 ) 1.42g, sodium chloride (NaCl) 8g, potassium chloride (KCl) 0.2g, add about 800mL of deionized water and stir to dissolve, then add concentrated hydrochloric acid to adjust the pH to 7.4, and finally dilute to 1L.
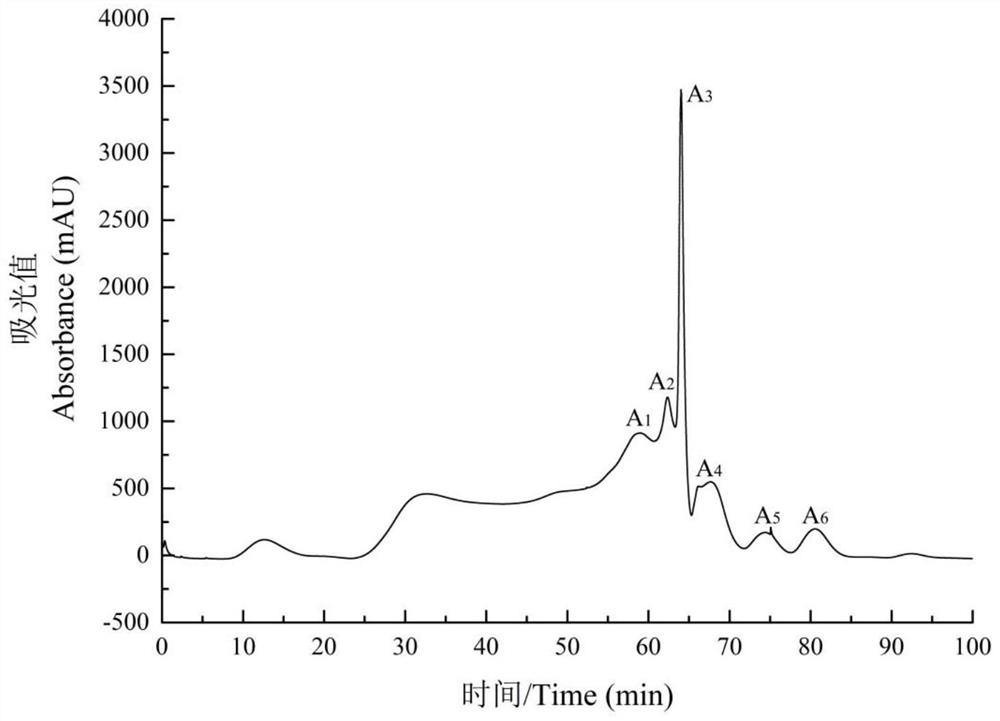
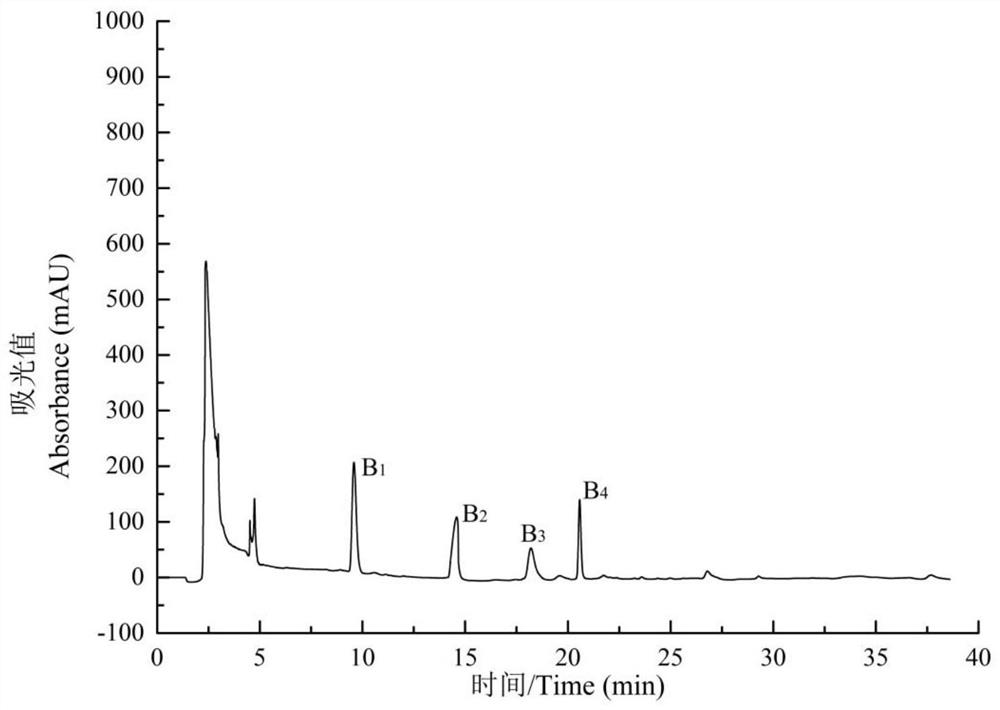
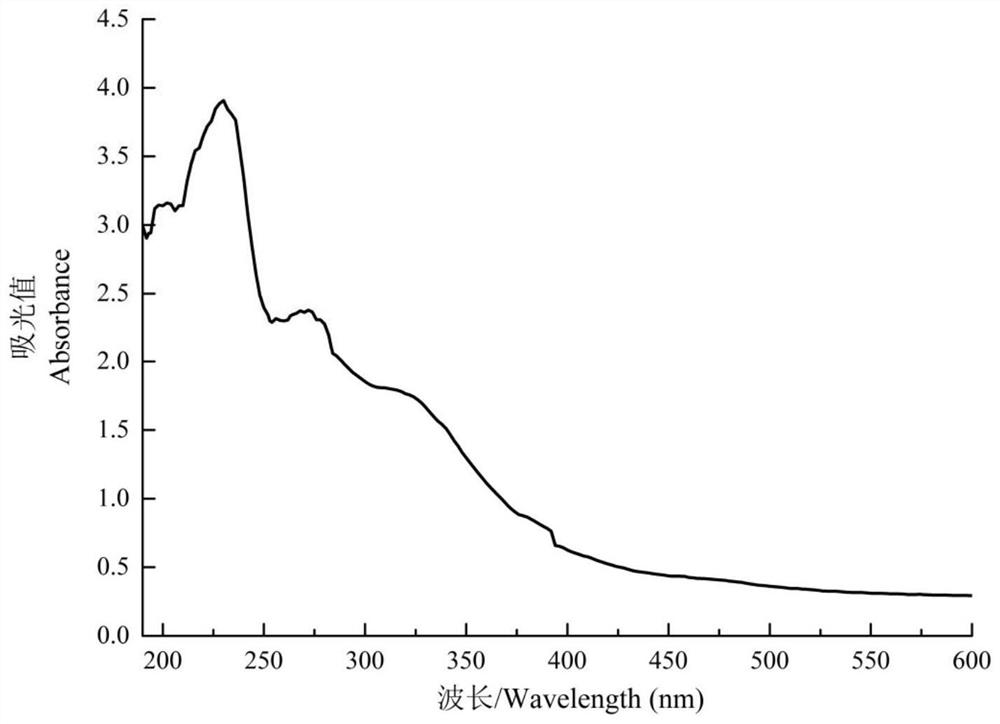
[0057] Add samples according to the ratio of solid to liquid of Morchella dry product / PBS buffer solution 1:20. After adding the sample, stir overnight with a magnetic stirrer at 120 rpm, and centrifuge at 5000 r / min to obtain the supernatant. Collect the supernatant, select a dialysis bag w...
Embodiment 3
[0063] Solid Phase Synthesis of Antioxidant Peptides from Morchella
[0064] Through LC-MS, we determined that the target polypeptide is composed of 12 amino acids and has a relative molecular mass of 1348Da. Now we are carrying out solid-phase synthesis to obtain morel-derived antioxidant peptides more efficiently.
[0065] Reagent materials required for solid-phase synthesis: 2-CL resin, amino acid (hereinafter expressed as Fmoc-aa-oh), dimethylformamide (DMF), dichloromethane (DCM), methanol, N,N'-diiso Propylcarbodiimide (DIC), N,N-Diisopropylethylamine (DIEA), 1-Hydroxybenzotriazole (HOBT), Trifluoroacetic Acid (TFA), Triisopropylsilane (TIS) , pure water, EDT, ether
[0066] Methods as below:
[0067] 1. Weigh 2g of 2-CL resin, put it into the reactor, soak it in DCM for 10min, wash it twice with DMF, then wash it once with DCM, and set it aside.
[0068] 2. Weigh the first (starting from the C-end) Fmoc-aa-oh (2g*0.3mmol / g*1.33=0.798 mmol), dissolve it with 5mL DCM, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com