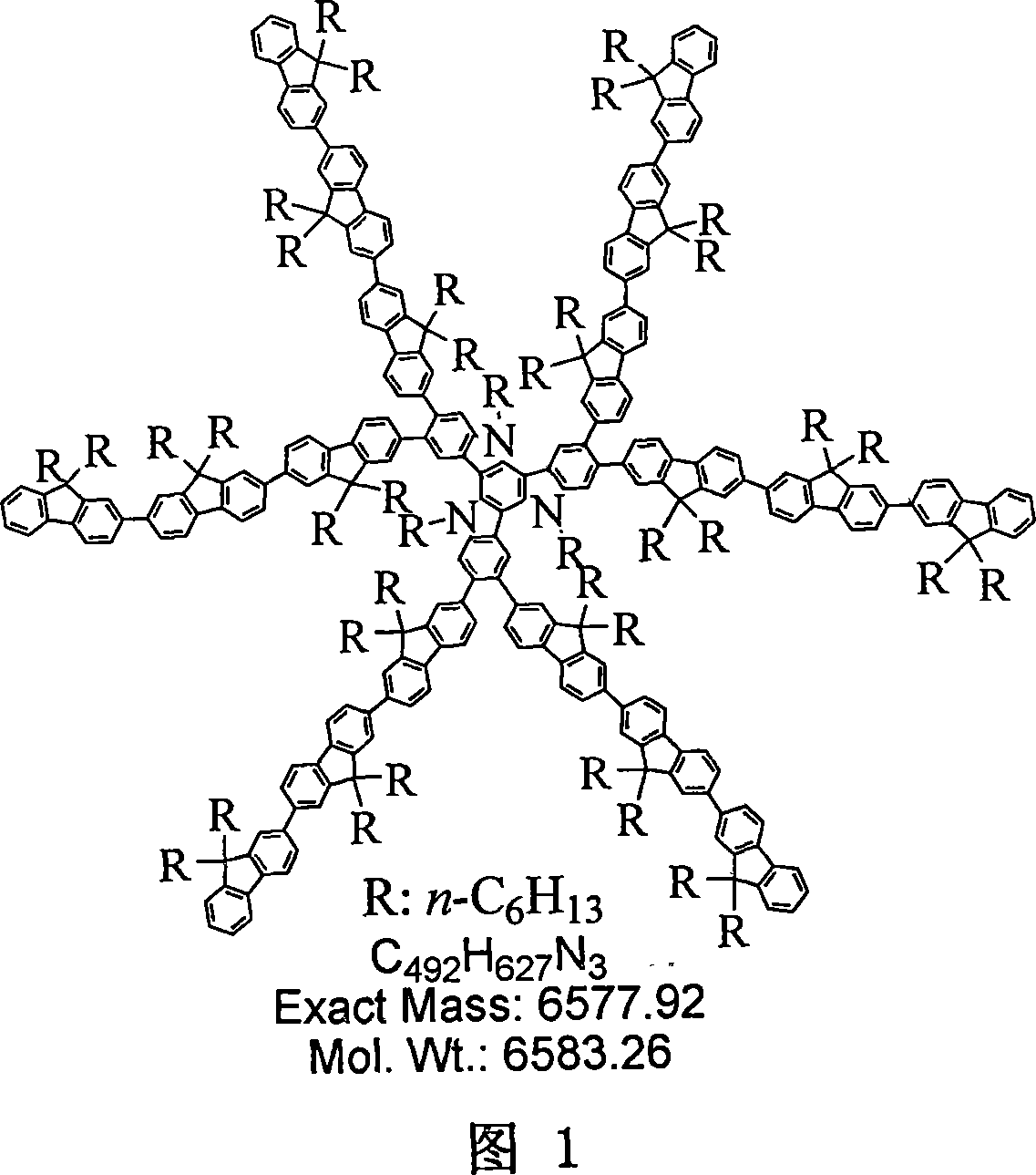
Branching structure functional material based on minami carbazole and preparation method and application thereof
A technology for arylation of trioxacarbazole and trioxacarbazole, which is applied in the field of six-arm or three-arm branched structural functional materials and their preparation, and achieves the effect of wide application value and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Embodiment 1: the synthesis of compound a-1
[0189] (1) Synthesis of Tricarbazole Precursor
[0190] Synthesis of Hexabromotricarbazole
[0191] Br 2 (22.44g, d=3.12g / ml, 7.19ml, 140.2mmol) was dissolved in acetocyanide (30ml), and added dropwise to the solution of indole (5.44g, 46.6mmol) in acetocyanide (100ml) for about 10min. Finish. The mixture was stirred overnight, and the obtained dark green precipitate was filtered, washed with a large amount of acetocyanide solution, and recrystallized in a DMSO / acetone mixed solution to obtain an off-white solid (3.21 g, 25.2%). 1 H NMR (400MHz, DMSO, δ, ppm): 12.13(3H, s), 8.88(3H, s), 7.87(3H, s).IR(KBr disc) / cm -1 : 3419.8, 2358.9, 1633, 1600, 1469, 1431, 1399, 1361, 1296, 1264, 1225, 1074, 1025, 949, 920, 866, 850, 701, 636, 529. Elemental analysis (%): Experimental determination: C 35.12, H 1.12, N 5.16; Theoretical: C 35.21, H 1.11, N 5.13.
[0192] Synthesis of Hexabromobutyl Tricarbazole
[0193] Hexabromotric...
Embodiment 2
[0203] The synthesis of embodiment 2 compound a-2
[0204] (1) Synthesis of hexabromohexyl tricarbazole
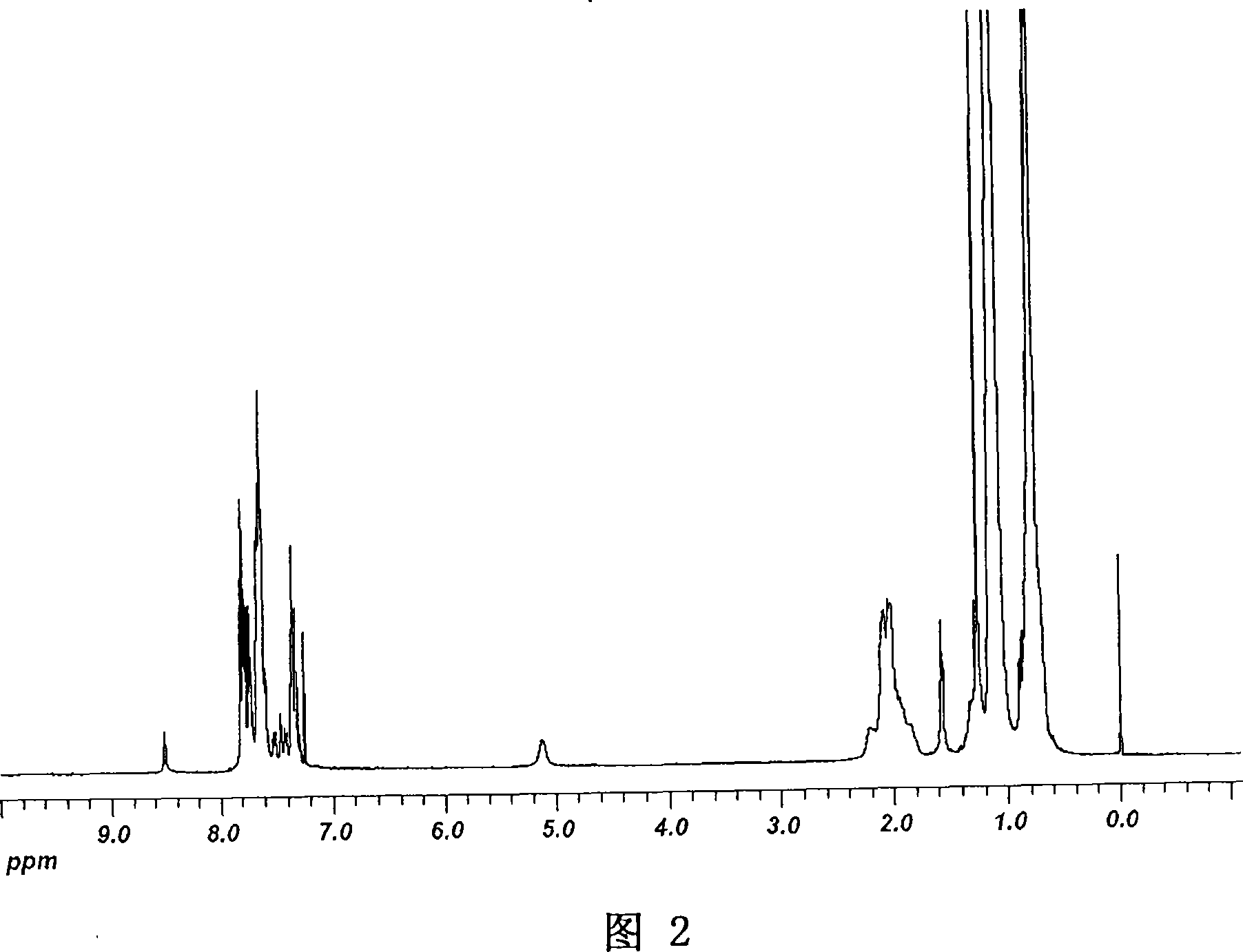
[0205] Synthesized according to the method in compound a-1, wherein the alkyl bromide can be 1-bromohexane, and the yield is 66%. 1 H NMR (400Hz, CDCl 3 , ppm, δ): 8.02 (s, 3H), 7.55 (s, 3H), 4.08 (m, 6H), 1.74 (brs, 6H), 1.33-1.26 (m, 18H), 0.87-0.84 (m, 9H ). 13 C NMR (100MHz, CDCl 3 , ppm, δ): 140.31, 138.93, 125.19, 123.13, 118.72, 115.43, 114.76, 101.53, 47.17, 31.70, 30.41, 26.41, 22.86, 14.24. MS (MALDI-TOF): Calcd for C 42 h 45 Br 6 N 3 : 1064.87; Found: 1069.0(M + ), 991.0 ([M-Br] + ), 913.1 ([M-2Br] + ), 833.2 ([M-3Br] + , 100%), 753.3 ([M-4Br] + ), 675.3 ([M-5Br] + ), 597.5 ([M-6Br] + ). Elemental analysis (%): experimental determination: C47.19 H 4.20, N, 3.89; theoretical value: C 47.09 H 4.23, N, 3.92.
[0206] (2) Synthesis of 9,9-dihexylfluorene monoboronic acid
[0207] Same as the synthesis of 9,9-dihexylfluorene monoboronic acid in compou...
Embodiment 3
[0211] The synthesis of embodiment 3 compound a-5
[0212] (1) Synthesis of hexabromophenylated tricarbazole
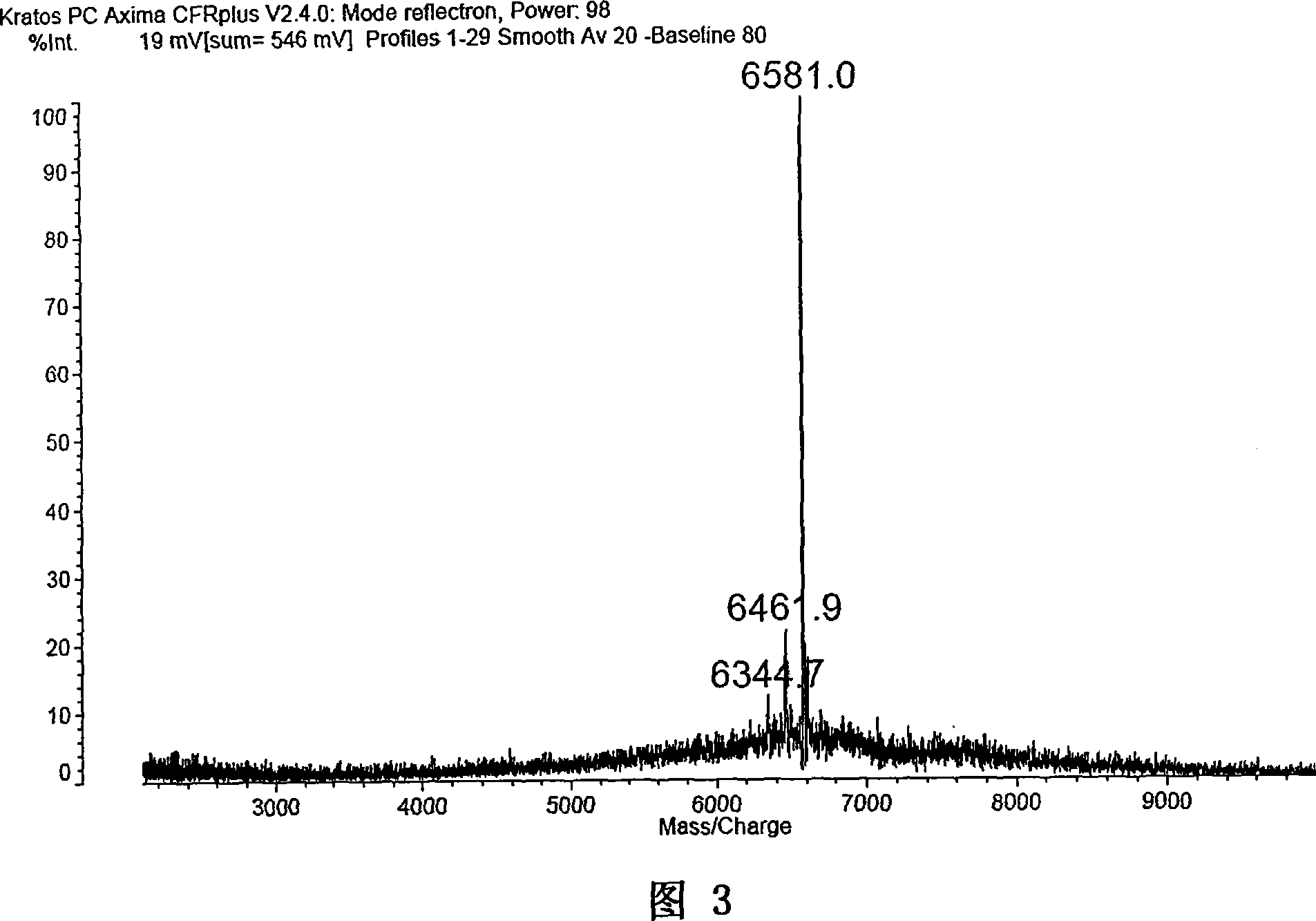
[0213] Hexabromotricarbazole (2.45g, 3mmol), 1-iodobenzene (2.75g, 13.5mmol), 1,10-phenanthroline (0.162g, 0.9mmol), CuI (98.5mg, 0.9mmol) and KOH (1.4g, 25mmol) was heated to reflux in a p-xylene (100ml) solution for 24h, and the obtained crude product was recrystallized in n-hexane and ethanol in turn to obtain a white crystal product (2.35g, 75%). 1 H NMR (400Hz, CDCl 3 , ppm, δ): 8.40(s, 3H), 7.71(s, 3H), 7.35-7.18(m, 15H). 13 CNMR (100MHz, CDCl 3 , ppm, δ): 141.2, 138.2, 129.4, 127.7, 125.6, 123.5, 121.6, 117.3, 116.9, 116.8, 114.8, 112.6. MS (MALDI-TOF): Calcd for C 42 h 21 Br 6N 3 : 1040.68; Found: 1047.0(M + ), 969.1 ([M-Br] + ), 891.3 ([M-2Br] + ), 813.4 ([M-3Br] + , 100%), 735.6 ([M-4Br] + ), 657.8 ([M-5Br] + ), 579.9 ([M-6Br] + ).
[0214] (2) Synthesis of 9,9-dihexylfluorene monoboronic acid
[0215] Same as the synthesis of 9,9-dihexylfluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com