Culture and use for dermis fibroblast
A technology of fibroblast-like cells and dermal cells, applied in tissue culture, animal cells, vertebrate cells, etc., can solve the problems of no direct evidence of multi-directional differentiation ability of clones, inability to effectively screen stem cells, and few clones.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] dermal fibroblast-like cells
[0088] 1. Take the remaining skin after circumcision, wash it with PBS, remove the subcutaneous tissue, and cut it to 2×2mm 2 size;
[0089] 2. 0.1% (w / v) neutral protease was digested overnight at 4°C, and the epidermis was removed, followed by 0.1% (w / v) type I collagenase, and digested at 37°C for 4 hours with constant temperature shaking;
[0090] 3. Collect the resulting cells at 1 x 10 3 / cm 2 inoculated on a petri dish at a density of 10% FCS high-sugar DMEM culture solution at 37°C (5% carbon dioxide);
[0091] 4. Change the medium 24-48 hours after inoculation to remove non-adherent cells, and change the medium every 3 days until the cells grow to 85% confluent and pass passage or perform corresponding detection.
Embodiment 2
[0093] detection
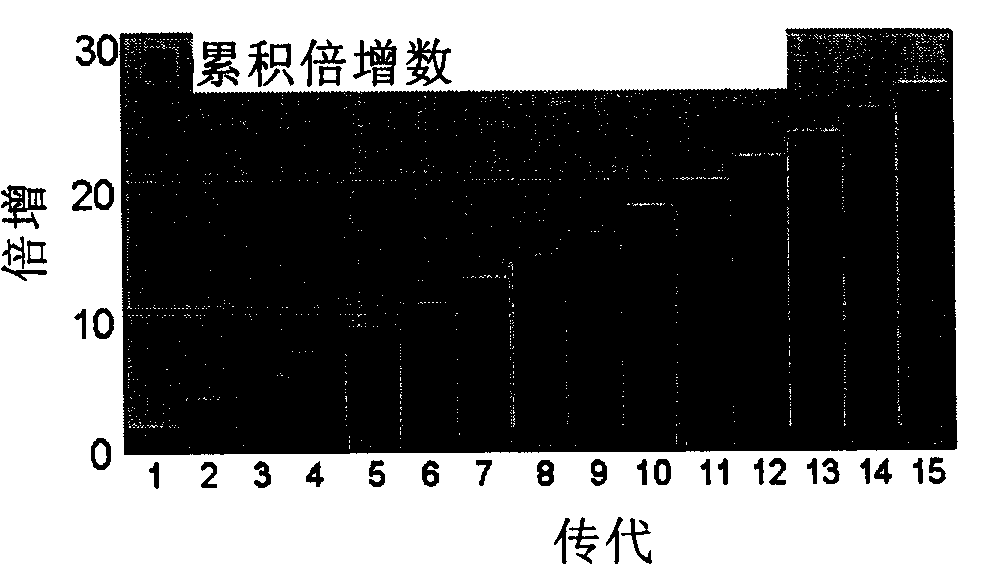
[0094] One, gained dermis cell in embodiment 1 with 3200 / cm 2 Inoculated in the cell culture plate at the density of the logarithmic growth phase, digested with 0.25% trypsin (containing 1mM EDTA) after the logarithmic growth phase, and continued to subculture to the 15th generation (about 97 days); the observation of the logarithmic growth phase used the MTT method (3 -[4,5-dimethylthia-zolyl-2]-2,5-diphenyltetrazolium bromide), the average MTT value from the 3rd day to the 12th day was continuously measured, and according to its growth curve, it was estimated that each generation of DDFCs grew to logarithmic proliferation The period of time (about 5-7 days), calculate the minimum doubling time. The specific formula is as follows: doubling time=growth time / doubling times; doubling times=(logN1-NO) / log2.
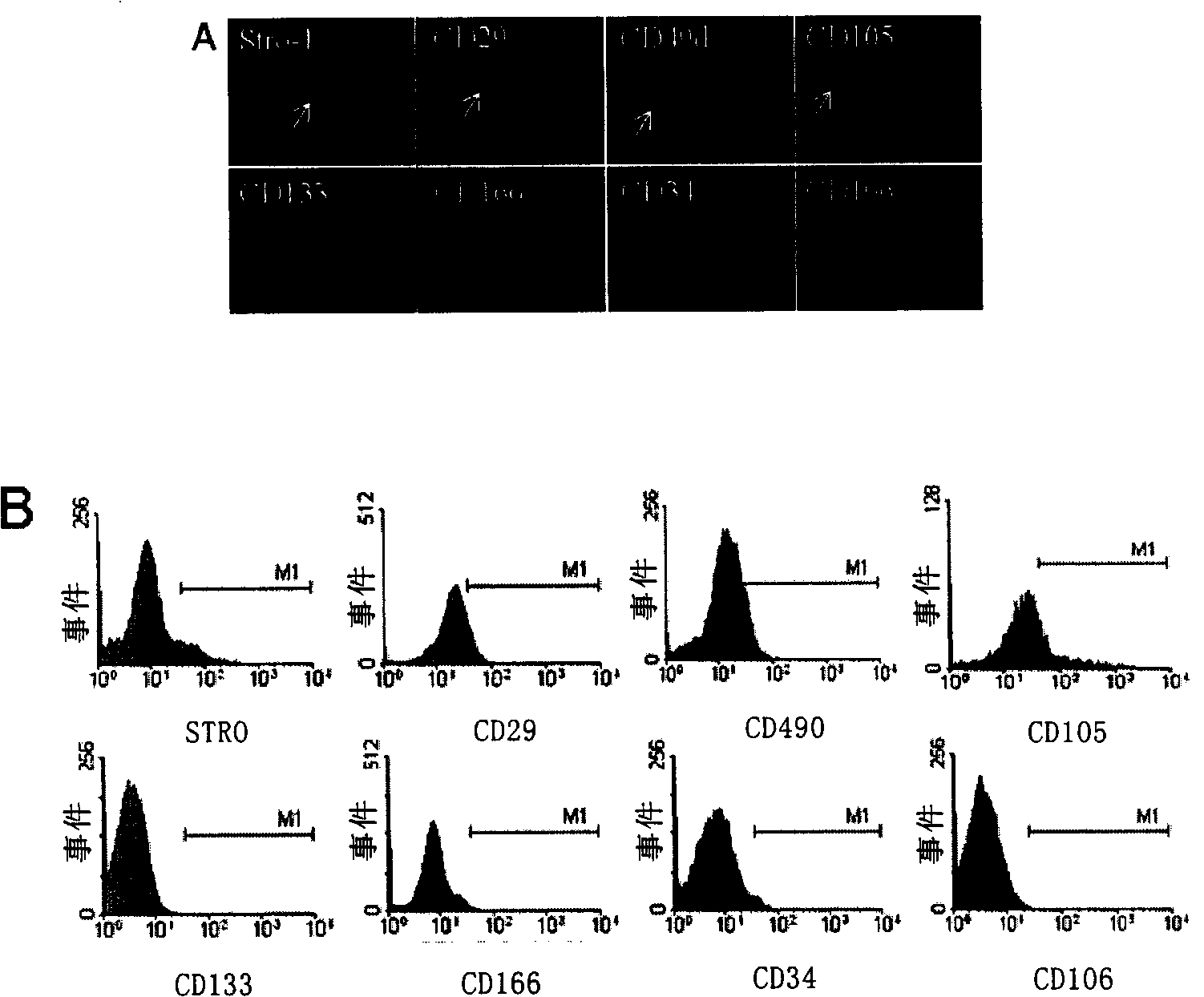
[0095] 2. Immunofluorescence detection
[0096] The dermis cells cultured on coverslips and various control cells were completely adhered to the wall a...
Embodiment 3
[0110] Induction and Detection of Adipogenicity of Dermal Fibroblast-like Cells
[0111] In Example 1, when the in vitro culture is nearly confluent, add adipose induction solution, which contains: DMEM culture solution, 10% FCS, 0.5mM isobutyl-methylxanthine (isobutyl-methylxanthine), 1 μM dexamethasone (dexamethasone), 10 μM Insulin (insulin), 200 μM indomethacin (indomethacin), the solution was changed every 3 days. After 3 weeks of induction, the cells were fixed with 4% paraformaldehyde and stained with Oil red. In order to detect the expression of fat-related genes PPAR-γ2 and Leptin, total cellular RNA was extracted and then detected by RT-PCR.
[0112] result
[0113] Three weeks after DDFCs were induced by adipogenicity, small vacuoles appeared in the cells, and the oil red staining was red, and there were no oil red staining cells in the uninduced group ( Figure 4 ); RT-PCR detection showed that 2 weeks after induction, the cells expressed adipocyte-specific gene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com