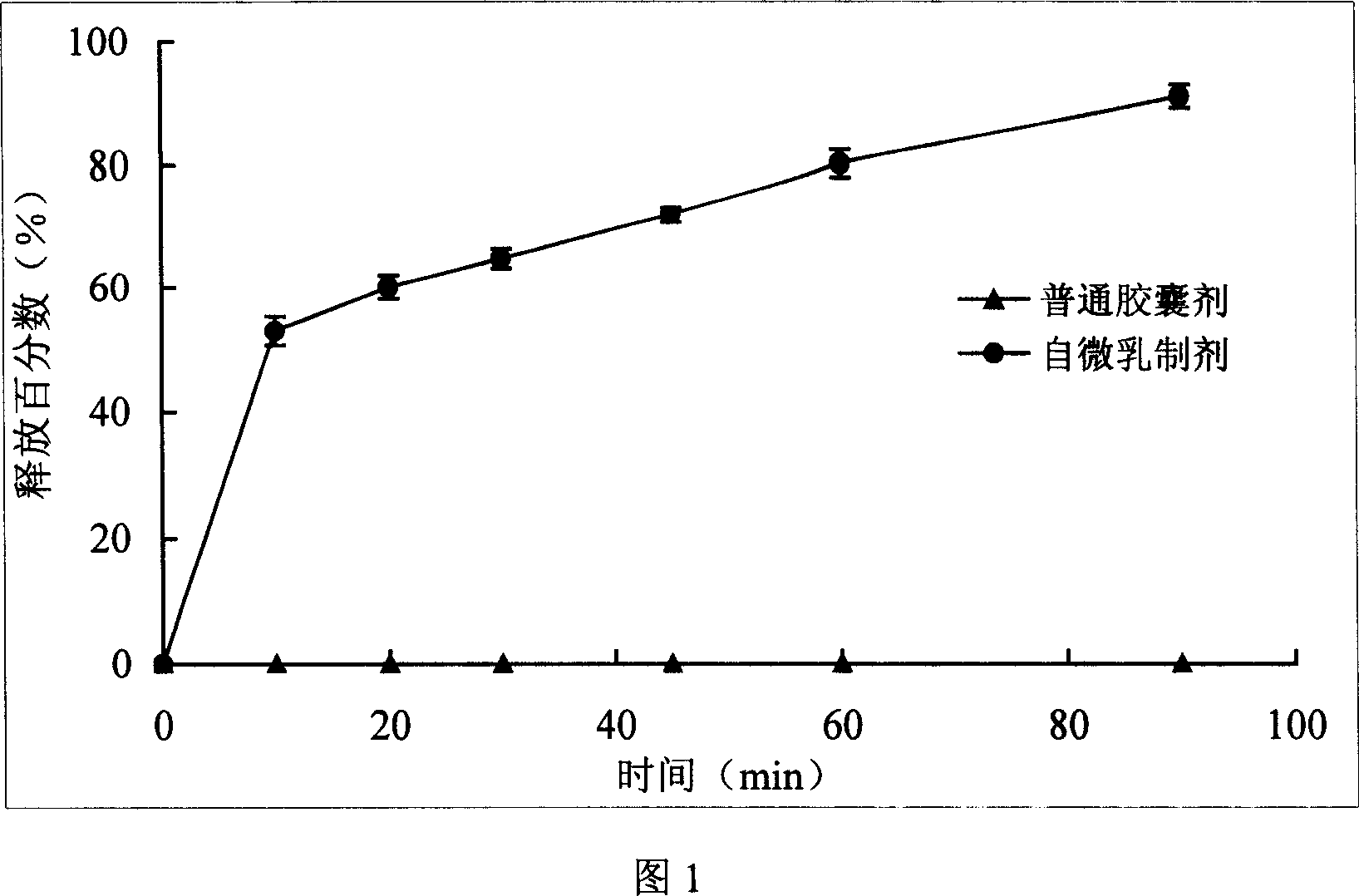
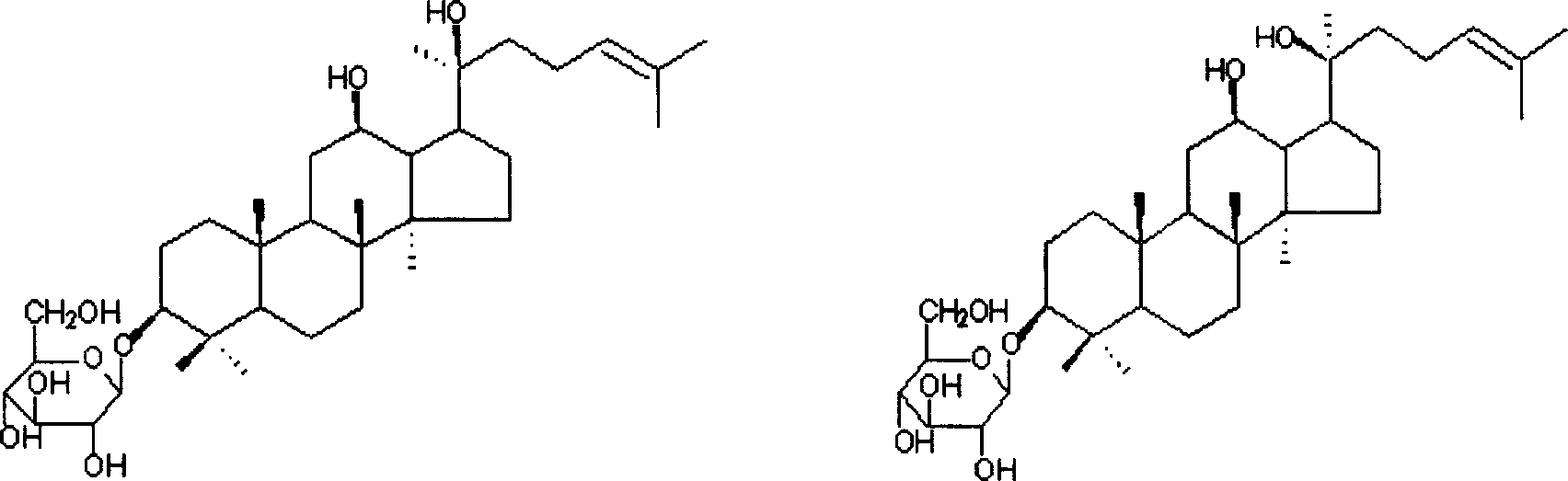
Ginsenoside Rh2 self-emulsifying composition and its preparation method
A technology of ginsenoside and self-microemulsion, which is applied in the field of self-microemulsion composition and preparation of the insoluble drug ginsenoside Rh2, can solve problems such as inconvenient use, toxic and side effects, and no ginsenoside research reports, and achieve Increased release rate, simple preparation method, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Take 10g of medium-chain fatty acid triacylglycerol (MCT) and 22g of polyoxyethylene castor oil (Cremophor EL), mix them evenly, put them in a water bath at 60°C and heat them, and take another 1g of ginsenoside Rh 2 Add it to 20g of ethylene glycol monoethyl ether (Transcutol P), ultrasonically disperse the drug evenly, add it to the above solution, and stir at 60°C for 2-3 hours until the drug is completely dissolved, which is obtained from microemulsion.
[0035] After the drug-loaded self-microemulsion was stored at 40°C for 3 months, ginsenoside Rh 2 The content of the microemulsion is 96.4%. Take 1 capsule and add 100 times of pure water to shake gently to form a microemulsion with a particle size of 28.0nm.
Embodiment 2
[0037] Add 1g of ginsenoside Rh2 to 26g of ethylene glycol monoethyl ether (Transcutol P), ultrasonically disperse the drug, heat it in a water bath at 60°C until the drug is completely dissolved, and take another 12g of Labrafil M 1944CS and 25g of polyoxyethylene castor oil (Cremophor EL) was added to the solution, stirred at a constant temperature of 60°C for 2-3 hours, and obtained from microemulsion.
[0038] After the drug-loaded self-microemulsion was stored at 40°C for 3 months, ginsenoside Rh 2 The content of the microemulsion is 96.7%. Take 1 capsule and add 100 times the amount of pure water to shake gently to form a microemulsion with a particle size of 89.7nm.
Embodiment 3
[0040] Take 10g Capryo190 and 15g polyoxyethylene castor oil (Cremophor EL), mix well, place in a 60°C water bath to heat, and take another 1g ginsenoside Rh 2 Add it to 30g of ethylene glycol monoethyl ether (Transcutol P), ultrasonically disperse the drug, add it to the above mixture, stir at a constant temperature of 60°C for 2-3 hours, and obtain it from the microemulsion, and prepare soft capsules from the microemulsion Back pack with enteric coating.
[0041] After the preparation was stored at 40°C for 3 months, ginsenoside Rh 2 The content of the microemulsion is 97.3%, and the particle size of the microemulsion formed after taking 1 capsule and adding 100 times the amount of pure water to shake gently is 31.7nm. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com