Oral insulin medicine and preparation method thereof
A technology for insulin and drugs, which is applied in the field of oral insulin drugs and its preparation, can solve the problems of intestinal mucosal cell damage, low bioavailability, and influence on use, and achieve the effects of good resistance to protease degradation, good dispersion performance, and easy absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
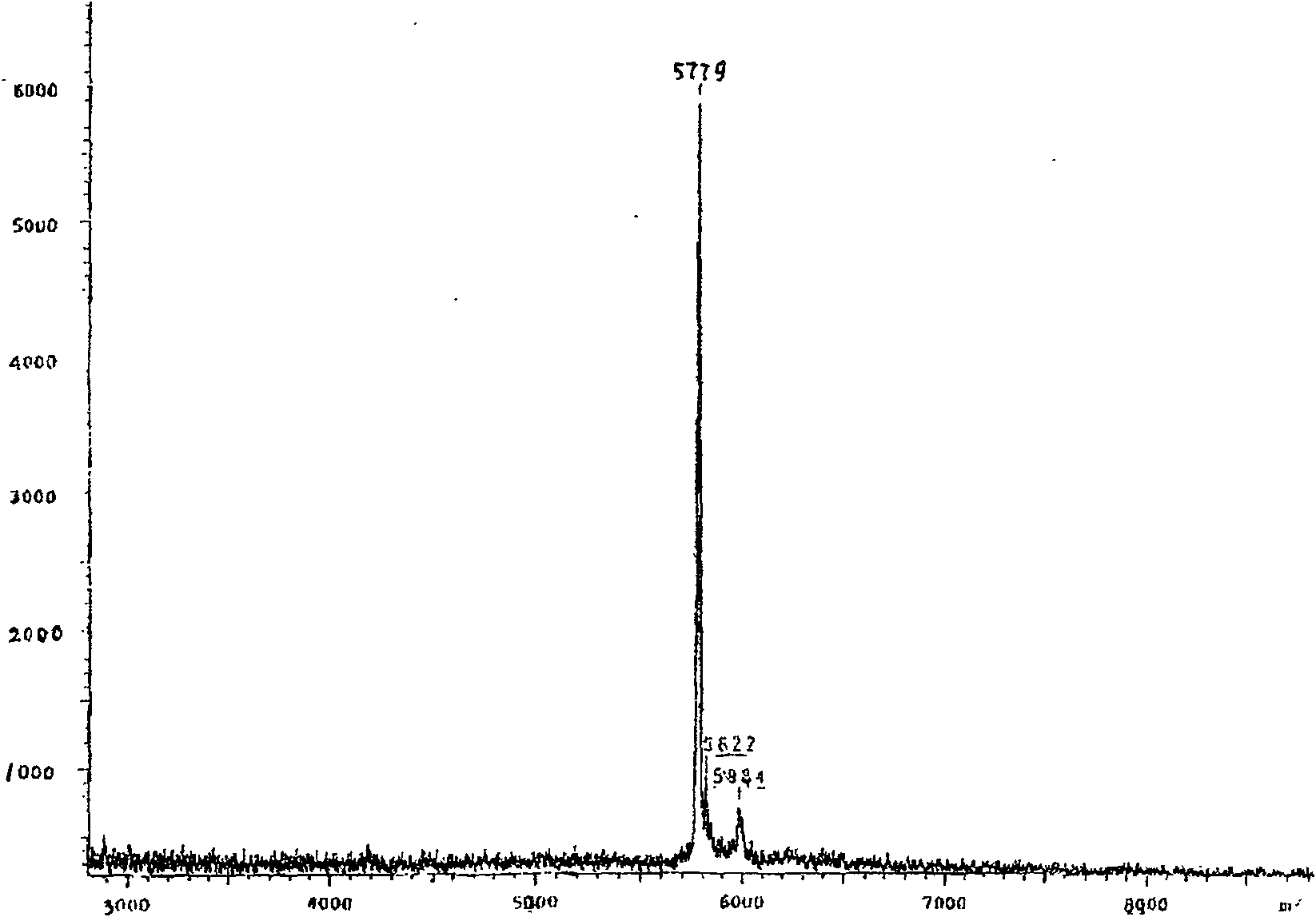
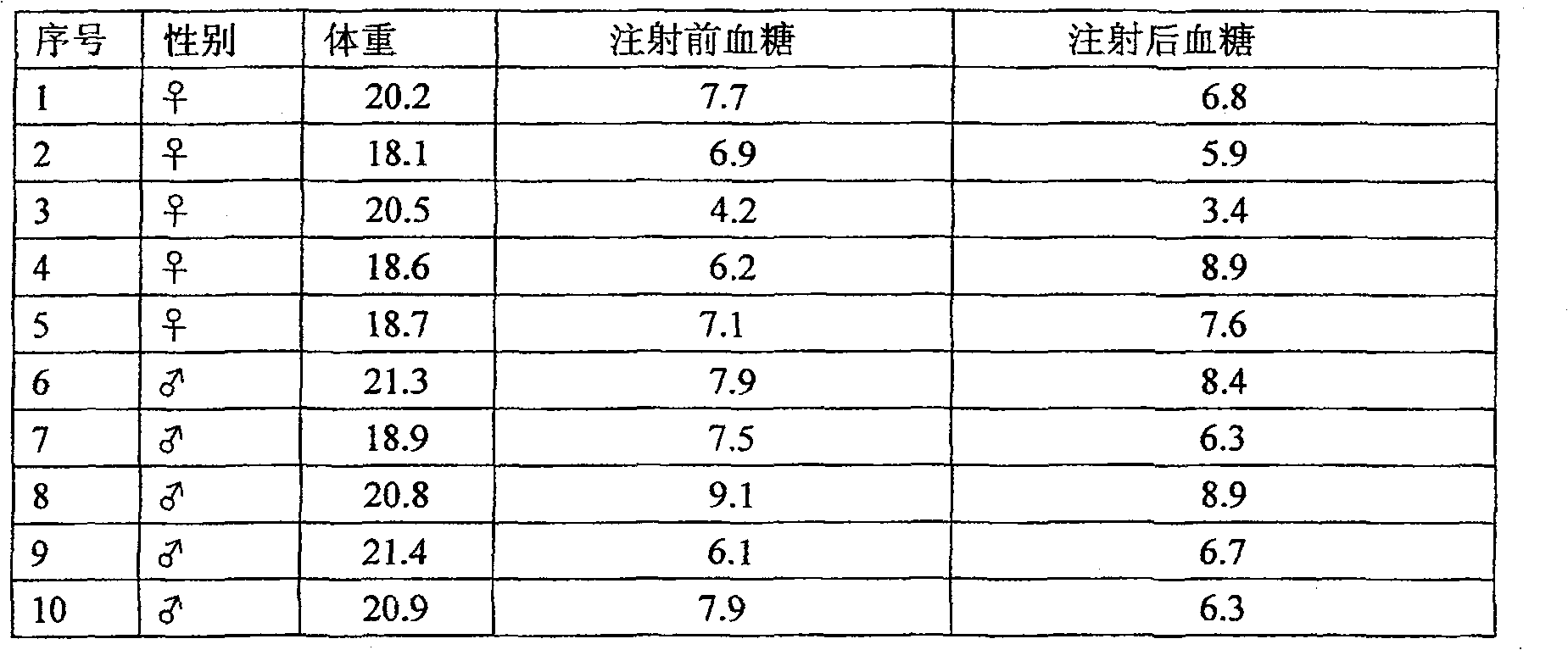
[0019] The activity test of the insulin extracted in the pig gallbladder bile of embodiment 1
[0020] 1. Confirmation of insulin and C-peptide in porcine bile by isotope radioimmunoassay
[0021] Animal specimens: take the bile of 1000 live pig gallbladders in fractions, use isotope radioimmunoassay method to detect the insulin and C-peptide in the bile, and take the average value as follows:
[0022] The average value of insulin in porcine gallbladder bile: 400±60iu / ml
[0023] The average value of radioactive C-peptide: 21±5ng / ml
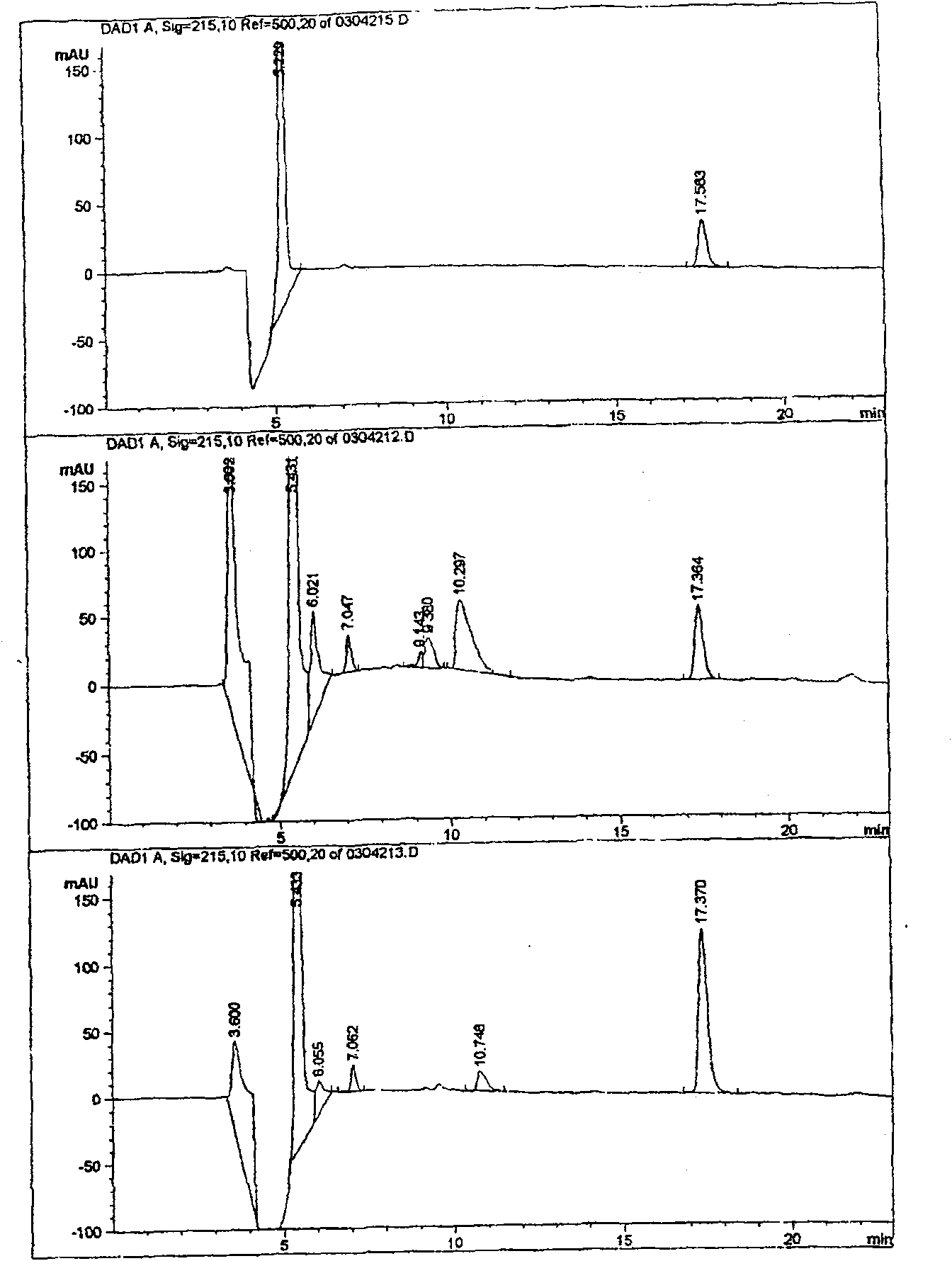
[0024] Two, the comparison between gallbladder bile extraction insulin high performance liquid chromatography separation figure and standard pure insulin (see figure 1 )
[0025] figure 1 The upper picture is the liquid chromatogram of standard pure insulin, the middle picture is the liquid chromatogram of insulin extracted from porcine gallbladder bile, and the lower picture is the liquid chromatogram of the mixture of standard pure insulin ...
Embodiment 2
[0045] Embodiment 2 Preparation of oral insulin medicine
[0046] Take egg yolk lecithin 200mg, sodium taurocholate 20mg, glycocholic acid 40mg, bilirubin 0.5mg, cholesterol 3mg, soak with 30% (v / v) ethanol solution 10-20ml, soak for 30 minutes, fully stir. Take 1 mg of insulin powder and dissolve it with a weak alkaline solution. After it is completely dissolved, add it to the above solution. Continue to stir, then add double-distilled water, continue to stir evenly, so that each ml of liquid contains 8U of insulin, and then put the solution in a water bath at 35-120°C to incubate 3 times, each time for about 1 hour, and the temperature of the water bath is gradually increased . After the liquid temperature drops to room temperature, add an appropriate amount of protamine. Place at 4°C overnight, centrifuge at 1000rmp for 5 minutes, discard the precipitate and keep the supernatant. Prepare a transparent, light yellow, low viscosity oral insulin drug solution.
Embodiment 3
[0047] The preparation of embodiment 3 oral insulin medicine
[0048] Take soybean lecithin 270mg, sodium taurocholate 28mg, glycocholic acid 120mg, bilirubin 1mg, cholesterol 2mg, soak with 10-20ml, 30% (v / v) ethanol solution for 30 minutes, fully stir. Take 1 mg of insulin powder and dissolve it with a weak alkaline solution. After it is completely dissolved, add it to the above solution. Continue to stir, then add double-distilled water, continue to stir evenly, so that each ml of liquid contains 8U of insulin, and then put the solution in a water bath at 35-120°C to incubate 3 times, each time for about 1 hour, and the temperature of the water bath is gradually increased . After the liquid temperature drops to room temperature, add an appropriate amount of protamine. Place at 4°C overnight, centrifuge at 1000rmp for 5 minutes, discard the precipitate and keep the supernatant. Prepare a transparent, light yellow, low viscosity oral insulin drug solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com