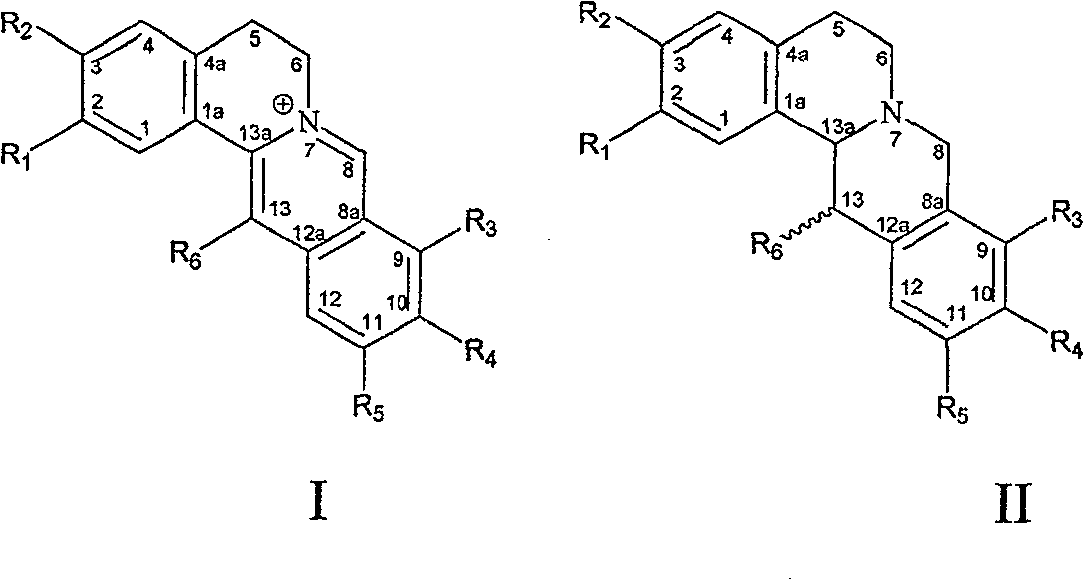
Use of protoberberine alkaloid in preparing against bacterial resistance medicament
A technology of original berberine and alkaloids, applied in antibacterial drugs, pharmaceutical formulas, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
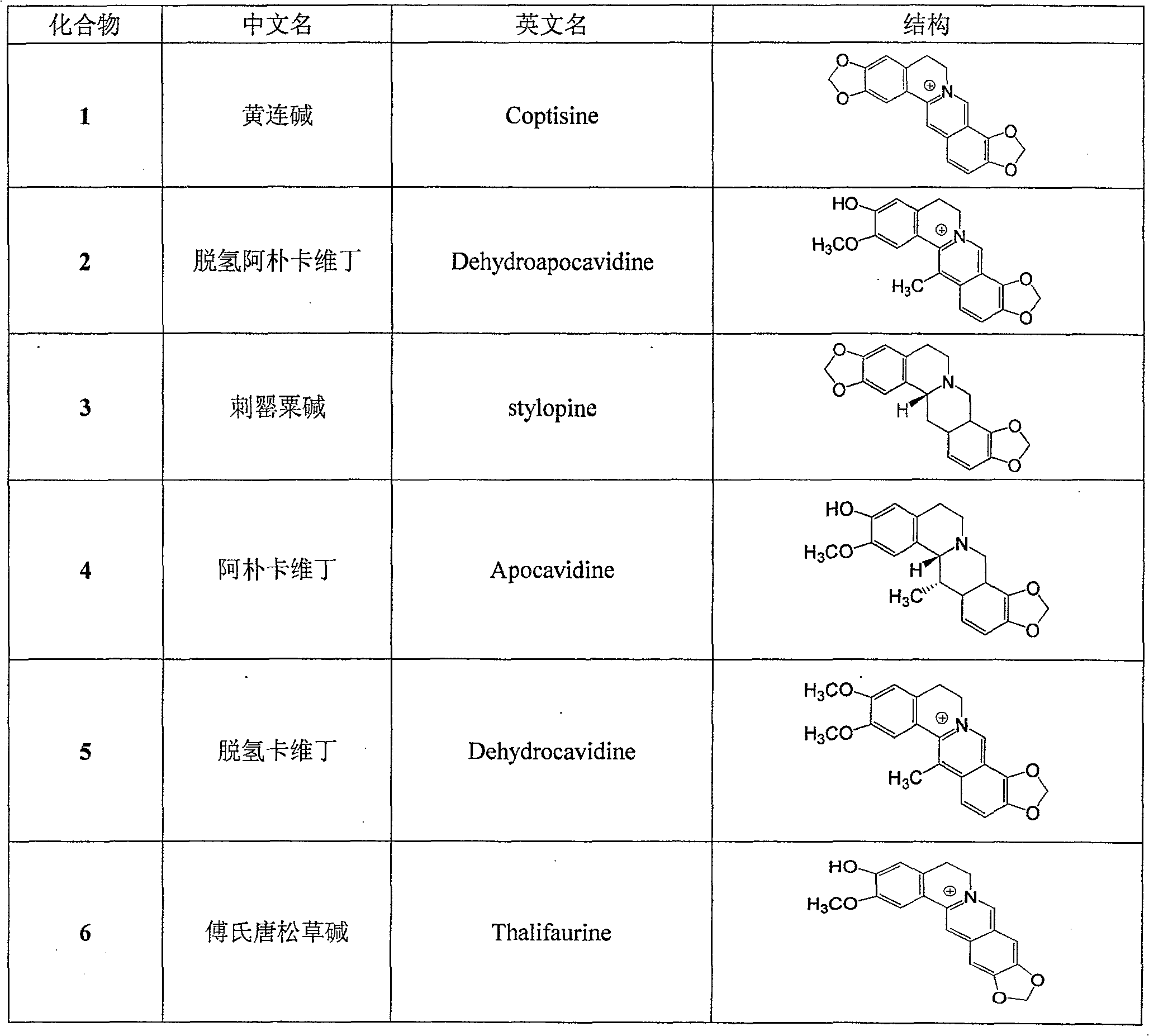
[0020] Example 1: Extraction and isolation of monomeric alkaloids 1 to 6 from the whole plant of Litectinus chinensis
[0021] Take 1 kg of dried whole herb powder of Corydalis saxicola Bunt., impregnate it with 5 L of 0.5% sulfuric acid at room temperature for 48 hours, and then filter it. Add sodium chloride to the filtrate until the concentration is 10%, and then adjust it to PH10 with saturated sodium hydroxide. Chloroform extraction, recovery of chloroform to obtain total alkali extract. This extract was stirred with acetone, left to stand, filtered, and the acetone was recovered to obtain 14 g of acetone soluble matter and 20 g of insoluble matter. The acetone soluble matter was subjected to low-pressure silica gel column chromatography, and gradient elution with petroleum ether:ethyl acetate=15:1~3:1 gave compounds 3 (0.08g), 4 (0.2g) and 5 (0.4g); Acetone insolubles were subjected to low-pressure silica gel column chromatography, and gradient elution with petroleum et...
Embodiment 2
[0022] Example 2: Physicochemical and spectral data of monomeric compounds 1 to 6
[0023] Coptisine (1)
[0024] yellow prismatic crystals, C 19 h 14 NO 4 + , mp 217-218°C. ESI MS (positive ion mode) m / z: 320. 1 H NMR (DMSO-d 6 , 400MHz): δ H 9.84 (1H, s, H-8), 8.90 (1H, s, H-13), 7.98 (1H, d, J=8.4Hz, H-11), 7.81 (1H, d, J=8.6Hz, H -12), 7.74 (1H, s, H-1), 7.00 (1H, s, H-4), 6.52 (2H, s, OC^O-9, 10), 6.11 (2H, s, OCH 2 0-2, 3), 4.79 (2H, dd, J=6.3, 5.9Hz, H-6), 3.19 (2H, dd, J=6.3, 6.0Hz, H-5); 13 C NMR (DMSO-d 6 , 100MHz): δ C105.1(d, C-1), 147.2(s, C-2), 149.6(s, C-3), 108.0(d, C-4), 130.4(s, C-4a), 26.1(t, C -5), 55.0(t, C-6), 143.9(d, C-8), 120.2(s, C-8a), 143.5(s, C-9), 147.4(s, C-10), 120.7 (d, C-11), 120.8 (d, C-12), 132.4 (s, C-12a), 121.5 (d, C-13), 136.7 (s, C-13a), 111.4 (s, C-13a), 111.4 (s, C- 13b), 101.8(t, OCH 2 O-2,3), 104.2(t, OCH 2 O-9, 10).
[0025] Dehydroapocavidin (2)
[0026] yellow amorphous solid, C 20 h 18 N0 4 + . ESI MS (po...
Embodiment 3
[0035] Embodiment 3: as the screening of the clinical drug-resistant strain MRSA of drug susceptibility test
[0036] 1. Test material
[0037] Antibiotic-sensitive paper sheets (China Institute for the Control of Pharmaceutical and Biological Products), MH agar (Hangzhou Tianhe Microbiological Reagent Co., Ltd.).
[0038] 2. Specimen source
[0039] Four bacterial strains were isolated and screened from clinical specimens in our hospital, numbered MRSA321, MRSA40, MRSA45, and MRSA55, and stored in the refrigerator at -20°C for later use.
[0040] 3. Bacteria identification
[0041] According to the "National Clinical Inspection Operation Regulations" and the bacterial identification method known to those skilled in the art, the above-mentioned sample bacteria are isolated and cultivated, and it is identified as Staphylococcus aureus (SA), and according to the known routine test method for antibiotic susceptibility (K-B Disc method), and the identification of drug-resistant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com