Method for preparing ferric lithium phosphate precursor by comprehensive utilization of ilmenite
A technology of lithium iron phosphate and ilmenite, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., to achieve simple process flow, stable product quality, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
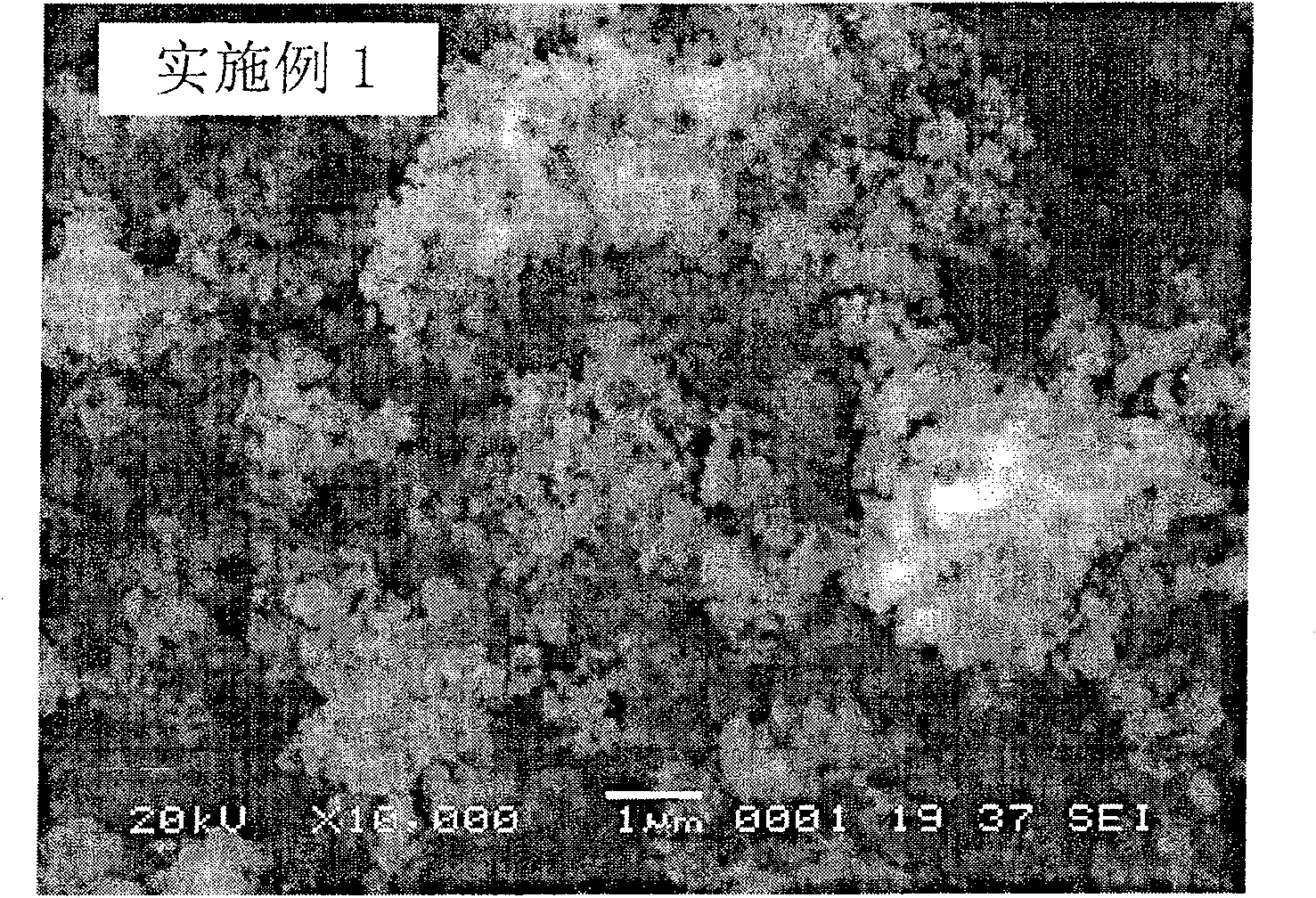
Embodiment 1
[0023] 500 grams of ilmenite are leached with sulfuric acid, filtered, and a certain amount of hematite and ferric sulfate are dissolved in the filtrate, so that the concentration of Fe in the mixed solution is 0.1mol / L, and the molar ratio of Ti to Fe is 0.3; Add a sufficient amount of sodium peroxide solution (1mol / L), then add phosphoric acid (1mol / L) that is equimolar to Fe, and adjust the pH to 2.5±0.1 with sodium hydroxide solution (0.5mol / L). React in a stirred reactor for 5 minutes, wash and filter the resulting precipitate, and dry it at 100°C to obtain a mixture of iron phosphate and doped phosphate, the precursor of lithium iron phosphate, the cathode material for lithium ion batteries.
Embodiment 2
[0025] Leach 500 grams of ilmenite with hydrochloric acid, filter, and dissolve a certain amount of ferric chloride in the filtrate, so that the concentration of Fe in the mixed solution is 1mol / L, and the molar ratio of Ti to Fe is 0.2; add a sufficient amount of Sodium hypochlorite solution (3mol / L), then add ammonium dihydrogen phosphate solution (0.1mol / L) equimolar to Fe, adjust pH=3.5±0.1 with lithium hydroxide solution (2mol / L), and stir at 60°C After reacting in the reactor for 24 hours, the resulting precipitate was washed, filtered, and dried at 50°C to obtain a mixture of iron phosphate and doped phosphate, which is the precursor of lithium iron phosphate, the positive electrode material of lithium ion batteries.
Embodiment 3
[0027] 500 grams of ilmenite are leached with sulfuric acid, filtered, and a certain amount of ferrous sulfate and ferrous chloride are dissolved in the filtrate, so that the concentration of Fe in the mixed solution is 2mol / L, and the molar ratio of Ti to Fe is 0.1; Add a sufficient amount of potassium chlorate solution (0.1mol / L) to the solution, then add an ammonium dihydrogen phosphate solution (3mol / L) equimolar to Fe, and adjust the pH to 4.5±0.1 with ammonia water (3mol / L). React in a stirred reactor for 10 hours, wash and filter the resulting precipitate, and dry it at 200°C to obtain a mixture of iron phosphate and doped phosphate, which is the precursor of lithium iron phosphate, the cathode material for lithium ion batteries.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com