Vegeta tive female sex hormone eudesmane compound, and its preparing method and use
The technology of a compound, eucalyptus, is applied in the field of natural medicinal chemistry and chemotherapeutics, which can solve the problem of weakening the protective effect of progesterone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation and isolation of total extracts
[0032] The rhizome of Cyperus rotundus contains a variety of eucalyptane sesquiterpenes. The dried and crushed rhizome of Cyperus cyperi (1kg) was soaked in methanol at room temperature and extracted completely (3 days / time, 8L / time×3). Concentrate the extract under reduced pressure to obtain methanol extract (132g). The extract is distributed with water-ethyl acetate, and the ethyl acetate layer is concentrated to obtain 65g of extract. The ethyl acetate extract contains various eucalyptus sesquiterpene components. The ethyl acetate extract was applied to a silica gel column and eluted with petroleum ether-ethyl acetate (100:1-0:100) gradient to obtain two fractions, F-1 and F-2, respectively. Among them, F-1 (about 2 g) was purified by silica gel column chromatography to obtain eucalyptus-4,11-dien-3-one. F-2 (about 3g) was decontaminated by cross-linked dextran LH-20, and then separated by silica gel column chromatograp...
Embodiment 2
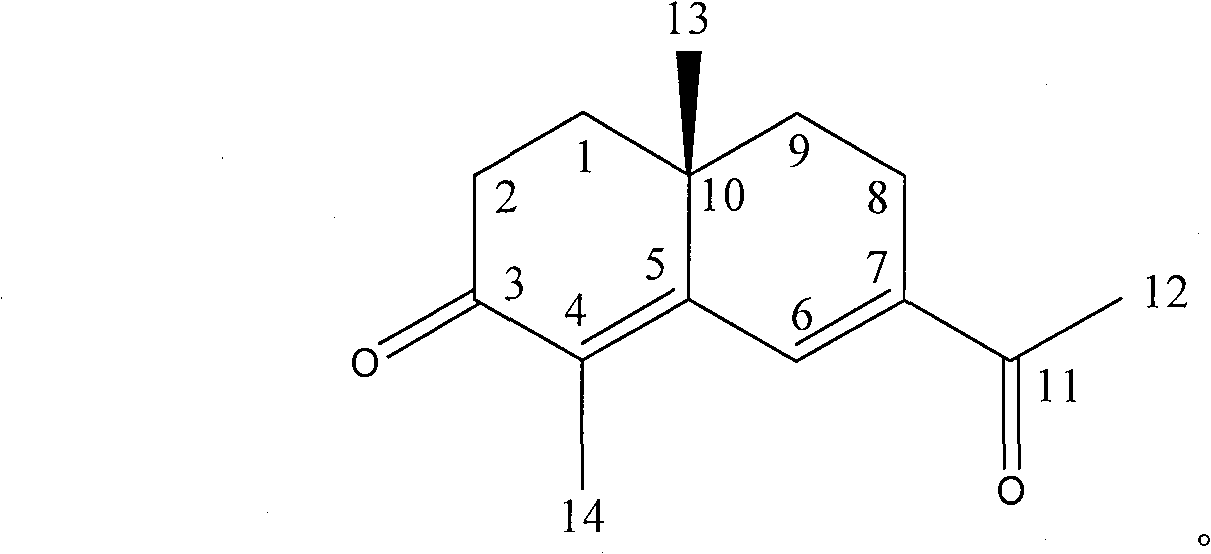
[0034] Separation and purification of norcine-4,6-diene-3,11-dione (4,6-Sueudesmadiene-3,11-dione).
[0035]
[0036] The ethyl acetate soluble part of the methanol extract of Rhizoma Cyperus was subjected to silica gel column chromatography, and the eluting part of petroleum ether-ethyl acetate (9:1) was taken, and impurities were removed by cross-linked dextran LH-20, and then purified by silica gel Repeated purification by column chromatography, eluting with petroleum ether-diethyl ether, gave a colorless oil. C 14 h 18 o 2 , HRESI[M+H] + m / z 219.1382, calculated 219.1385. [α] D +255°. Soluble in chloroform.
[0037] 1 H NMR (CDCl 3 , 400MHz): δ7.44 (1H, s, H-6), 2.43 (3H, s, H-12), 1.96 (3H, s, H-14), 1.10 (3H, s, H-13); 13 C NMR (CDCl 3 , 100MHz): 198.9(s, C-3), 198.8(s, C-11), 153.0(s, C-5), 143.1(s, C-7), 133.2(s, C-4), 132.4 (d, C-6), 36.3 (t, C-2), 36.2 (t, C-9), 33.9 (t, C-1), 33.1 (s, C-10), 25.8 (q, C- 12), 21.2 (q, C-13), 20.9 (t, C-8), 10.8 (q, C-...
Embodiment 3
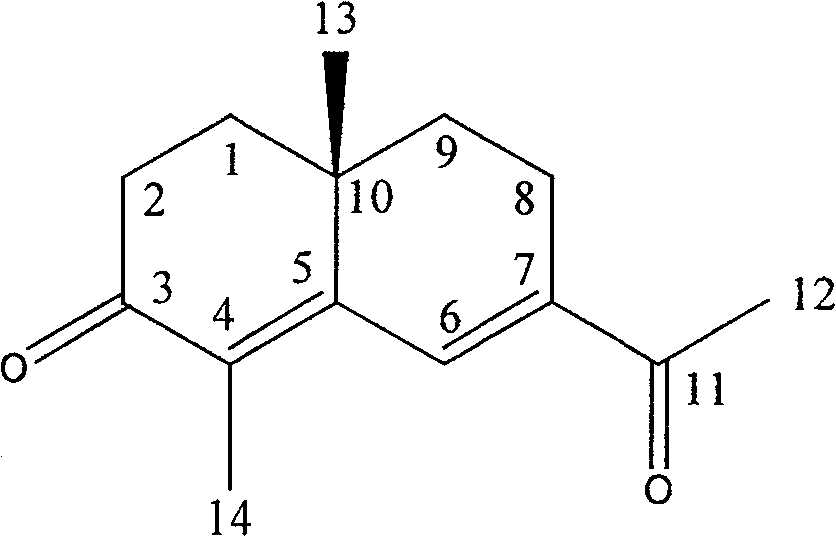
[0039] Separation and Purification of Eudesmadieh-3-one (4,11-Eudesmadieh-3-one)
[0040] The ethyl acetate soluble part of the methanol extract of Rhizoma Cyperus was subjected to silica gel column chromatography, eluted with petroleum ether-ethyl acetate, and repeatedly purified to obtain a colorless oily substance. C 15 h 22 O, easily soluble in chloroform.
[0041] 1 H NMR (CDCl 3 , 400MHz): δ4.78 (2H, s, H-12), 2.74 (2H, q, J=1.7 Hz, H-6), 2.52 (2H, q, J=6.5Hz, H-2), 2.43 (2H, t, J=6.5Hz, H-2), 2.08(2H, d, J=8.9Hz, H-6), 1.78(6H, s, H-13, H-14), 1.24(3H, s, H-15).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com