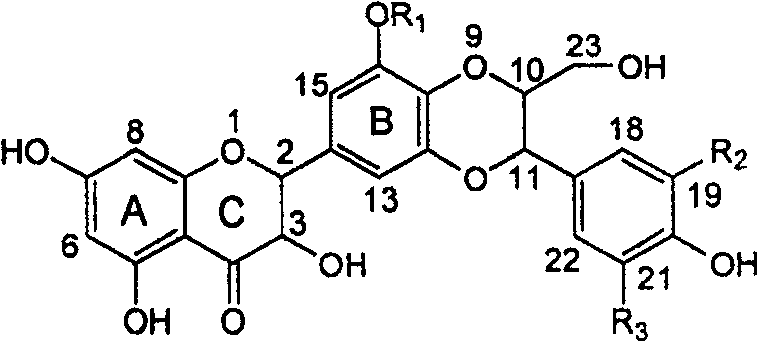
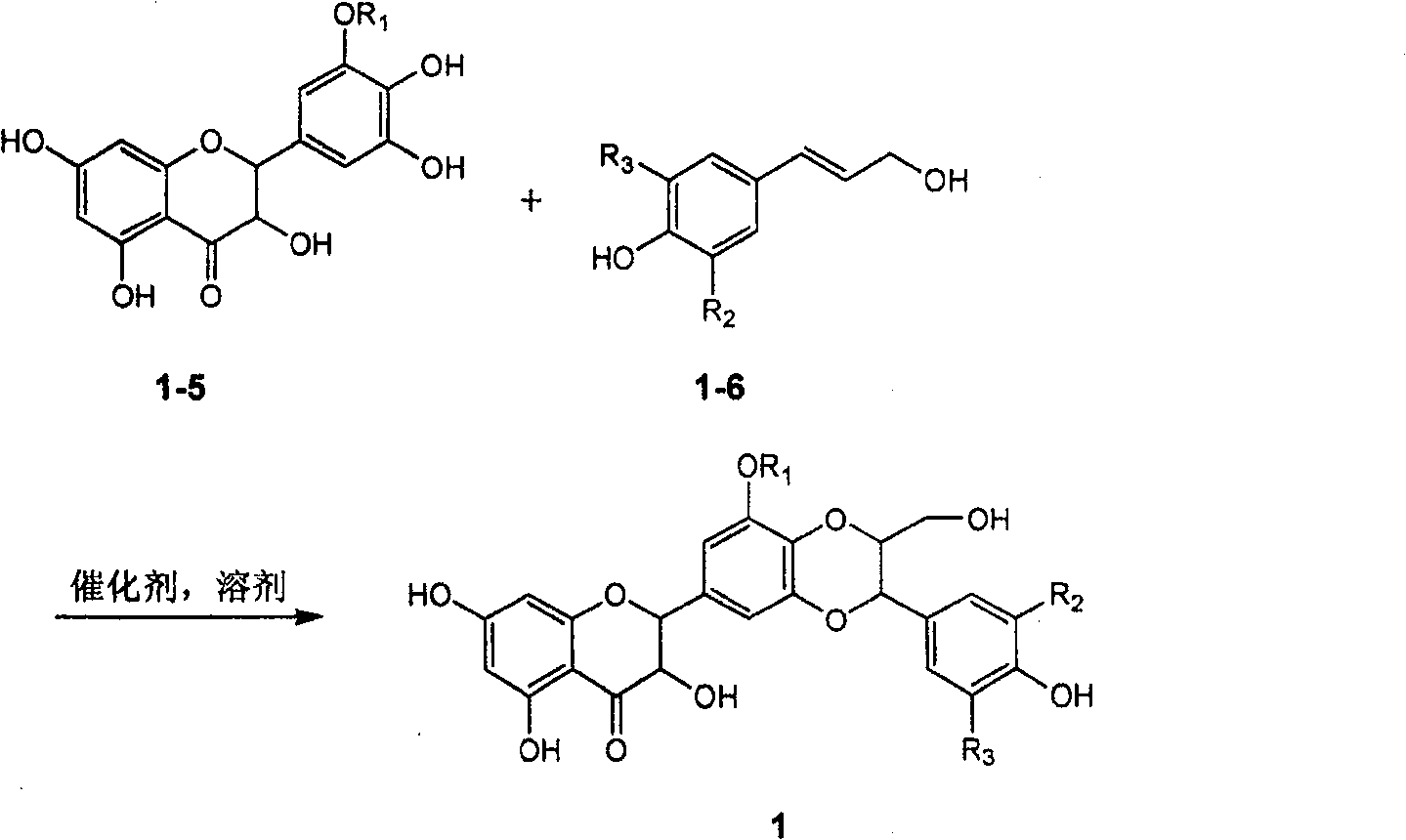
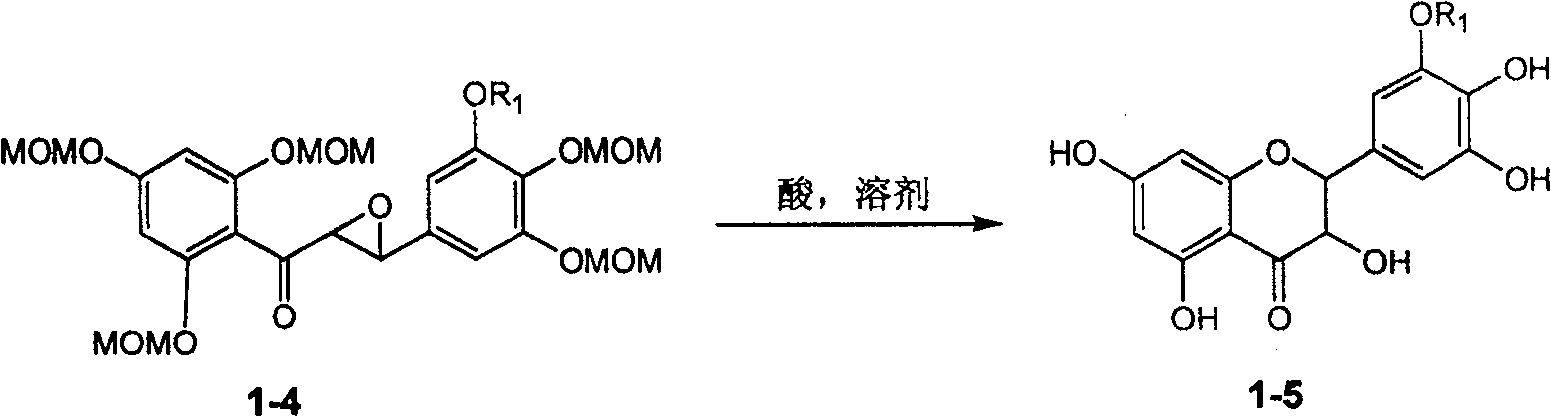
Silybin flavonolignan and their production method and use
A technology of lipids and medicinal salts, applied in the field of organic chemistry and medicinal chemistry, can solve problems such as insufficient water solubility and bioavailability, and limited drug market
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Compound 1-3-1 (1-(2,4,6-trimethoxymethoxyphenyl)-3-(3,4-dimethoxymethoxy-5-methoxyphenyl ) propenone) preparation:
[0040]
[0041] 3 grams of 3-methoxy-4,5-dimethoxymethoxybenzaldehyde and 3.5 grams of 2,4,6-trimethoxymethoxyacetophenone were dissolved in 40mL of ethanol, and 10g of potassium hydroxide was added 20 mL of aqueous solution was stirred at room temperature for 15 hours. Ethanol was distilled off under reduced pressure, 30 mL of water was added, and extracted with ethyl acetate (3×20 mL). After combining the organic phases, they were washed successively with saturated sodium bisulfite (40 mL) and saturated brine (40 mL), dried over anhydrous sodium sulfate, concentrated by filtration, and obtained 4.4 g of compound 1-3-1 yellow oil by column chromatography. Yield 70%.
[0042] R f (PET / EtOAc=2:1)0.36; UV(MeOH)λ max =208,321nm; 1 H NMR (400MHz, deuterated chloroform) δ: 3.34(s, 6H, OCH 3 ), 3.43 (s, 3H, OCH 3 ), 3.46(s, 3H, OCH 3 ), ...
Embodiment 2
[0044] Example 2: Compound 1-3-2 (1-(2,4,6-trimethoxymethoxyphenyl)-3-(3,4-dimethoxymethoxy-5-ethoxyphenyl ) propenone) preparation:
[0045]
[0046] R f (PET / EtOAc=2:1)0.40; UV(MeOH)λ max =210,325nm; 1 H NMR (400MHz, deuterated chloroform) δ: 1.39(t, J=7.2Hz, 3H, CH 3 ), 3.34(s, 6H, OCH 3 ), 3.43 (s, 3H, OCH 3 ), 3.46(s, 3H, OCH 3 ), 3.56(s, 3H, OCH 3 ), 4.02 (q, J=7.2Hz, 2H, CH 2 ), 5.06(s, 4H, OCH 2 O), 5.11(s, 4H, OCH 2 O), 5.14(s, 2H, OCH 2 O), 6.51(s, 2H, H-3', 5'), 6.74(d, J=1.6Hz, 1H, H-2), 6.80(d, J=16.0Hz, 1H, H-α), 6.91(d, J=1.6Hz, 1H, H-6), 7.17(d, J=16.0Hz, 1H, H-β); ESI-MS: 553(M+1) + .
Embodiment 3
[0047] Example 3: Compound 1-4-1 (1-(2,4,6-trimethoxymethoxyphenyl)-3-(3-methoxy-4,5-dimethoxymethoxyphenyl ) the preparation of propylene oxide):
[0048]
[0049] Dissolve 3 g of compound 1-3-1 in 50 ml of methanol, add 4 mL of 2N sodium hydroxide and 4 mL of 30% hydrogen peroxide under stirring, stir at room temperature for 10 hours, evaporate the solvent under reduced pressure, add 50 mL of water, acetic acid Ethyl ether extraction (3 x 30 mL). The organic phases were combined, washed with saturated brine (40 mL), and dried over anhydrous sodium sulfate. Filtration and concentration gave 2.6 g of yellow oil with a yield of 85%, which could be directly used in the next reaction.
[0050] R f (PET / EtOAc=2:1)0.37; UV(MeOH)λ max =205,278nm; 1 H NMR (400MHz, deuterated chloroform) δ: 3.34(s, 6H, OCH 3 ), 3.43 (s, 3H, OCH 3 ), 3.46(s, 3H, OCH 3 ), 3.56(s, 3H, OCH 3 ), 3.87 (s, 3H, OCH 3 ), 3.89(d, J=1.6Hz, 1H, H-β), 3.93(d, J=1.6Hz, 1H, H-α), 5.06(s, 4H, OCH 2 O), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com