Quality control method of ginkgo oral carity disintegration tablet
The technology of an orally disintegrating tablet and a detection method is applied in the quality control of ginkgo orally disintegrating tablets and the field of medicines, and can solve the problem of the quality control method of ginkgo orally disintegrating tablet, inaccurate detection, and inability to solve the quality control method well. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] 1000 orally disintegrating tablets formula:
[0101] Ginkgo Biloba Extract 150g
[0102] Ethyl cellulose 20g
[0103] Mannitol 58g
[0104] Microcrystalline Cellulose 40g
[0105] Croscarmellose sodium (CCN z ) 16g
[0106] Citric acid 5g
[0107] Sodium bicarbonate 5g
[0108] Aspartame 2g
[0109] Peppermint essence 2g
[0111] Quality control methods are as follows:
[0112] Properties: This product is an orally disintegrating tablet, the drug is white and brown spots, and can disintegrate rapidly in the mouth without obvious bitterness and gritty feeling;
[0113] Identification includes:
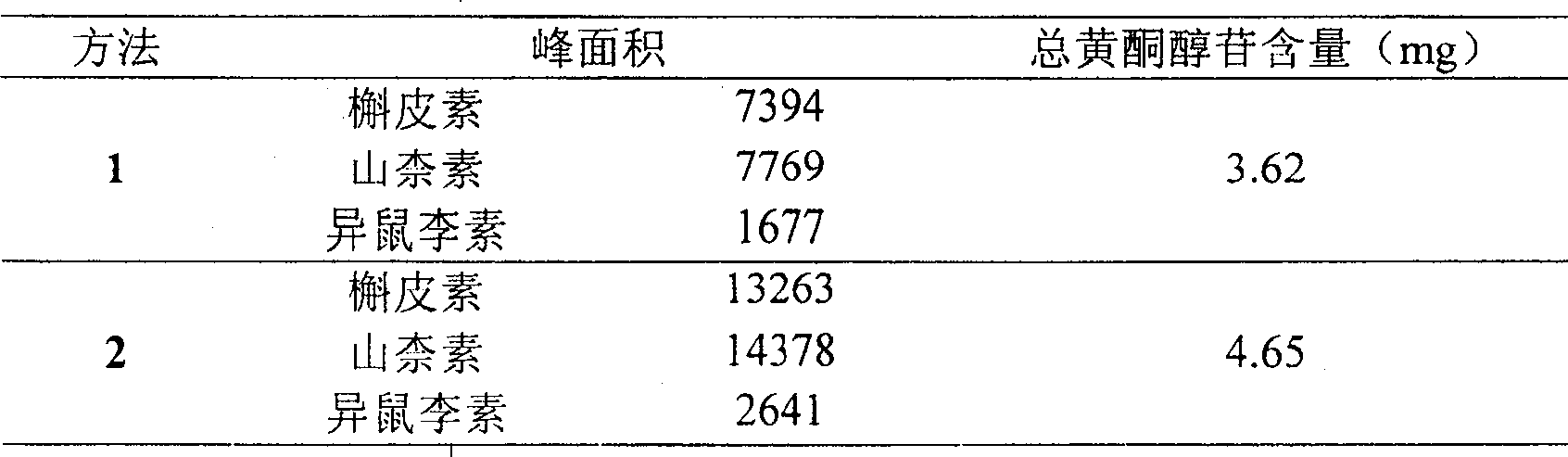
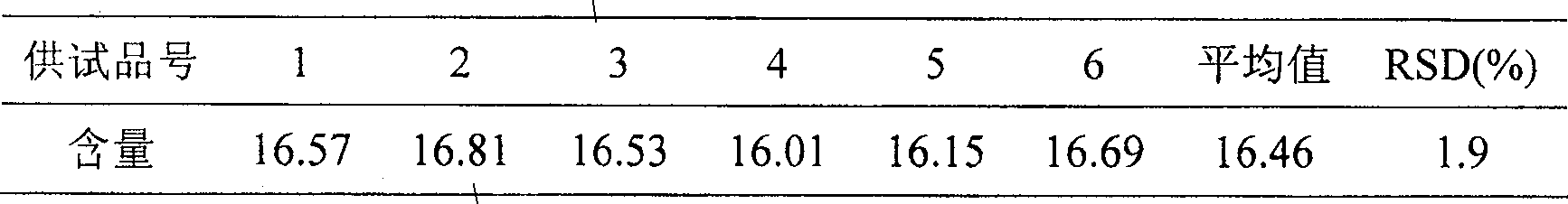
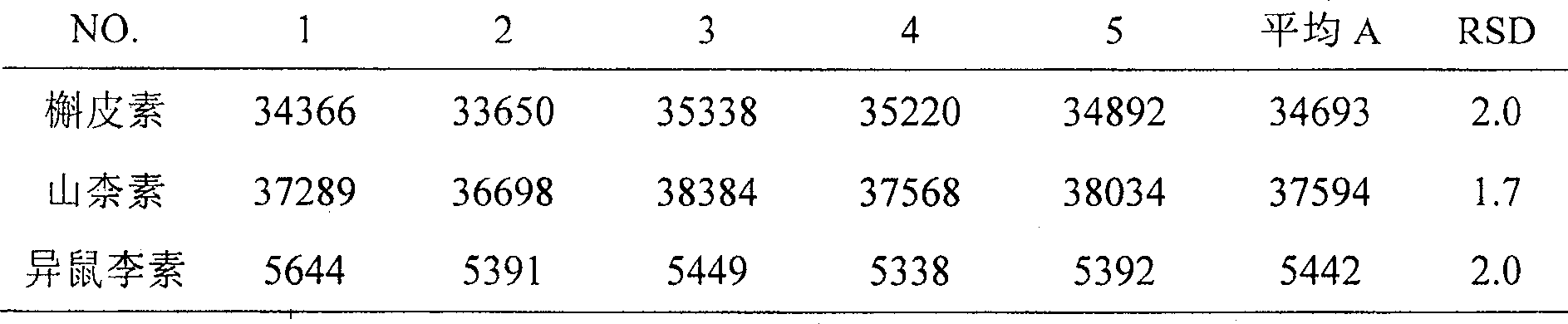
[0114] (1) Take this product, approximately equivalent to 30 mg of total flavonol glycosides, grind it finely, add 20 ml of a mixed solution of 25% HCL:methanol=1:4, heat and reflux for 1 hour, filter, add 30 ml of distilled water to the filtrate, and then Evaporate to 20ml, extract 3 times with ether, 15ml each time, combine the ether s...
Embodiment 2
[0125] 1000 orally disintegrating tablets formula:
[0126] Ginkgo Biloba Extract 40.0g
[0127] Polyacrylic resin IV 10.0g
[0128] Ethyl cellulose 10.0g
[0129] Mannitol 100.0g
[0130] Sodium starch glycolate 2.0g
[0131] Cross-linked polyvinylpyrrolidone 20.0g
[0132] Low-substituted hydroxypropyl cellulose 25.0g
[0133] Microcrystalline Cellulose 15g
[0134] Aspartame 5.0g
[0135] Micronized silica gel 10g
[0136] Magnesium Stearate 2.5g
[0137] Quality control methods are as follows:
[0138] Properties: This product is an orally disintegrating tablet, the drug is white and brown spots, and can disintegrate rapidly in the mouth without obvious bitterness and gritty feeling;
[0139] Identification includes:
[0140] (1) Take this product, approximately equivalent to 50 mg of total flavonol glycosides, grind it finely, add 100 ml of a mixed solution of 50% HCL:methanol=9:1, heat and reflux for 5 hours, filter, add 50 ml of distilled water to the filtrat...
Embodiment 3
[0151]1000 orally disintegrating tablets formula:
[0152] Ginkgo Biloba Extract 100g
[0153] Ethylcellulose 15g
[0154] Mannitol 50g
[0155] Microcrystalline Cellulose 35g
[0156] Croscarmellose sodium (CCN z ) 10g
[0157] Citric acid 5g
[0158] Sodium bicarbonate 5g
[0159] Aspartame 5g
[0160] Peppermint essence 3g
[0161] Micronized silica gel 10g
[0163] Quality control methods are as follows:
[0164] Properties: This product is an orally disintegrating tablet, the drug is white and brown spots, and can disintegrate rapidly in the mouth without obvious bitterness and gritty feeling;
[0165] Identification includes:
[0166] (1) Take this product, approximately equivalent to 10 mg of total flavonol glycosides, grind it finely, add 10-ml of a mixed solution of 10% HCL: methanol = 1:9, heat and reflux for 0.5 hours, filter, add 10 ml of distilled water to the filtrate , then evaporated to 5ml, extracted once with ether,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com