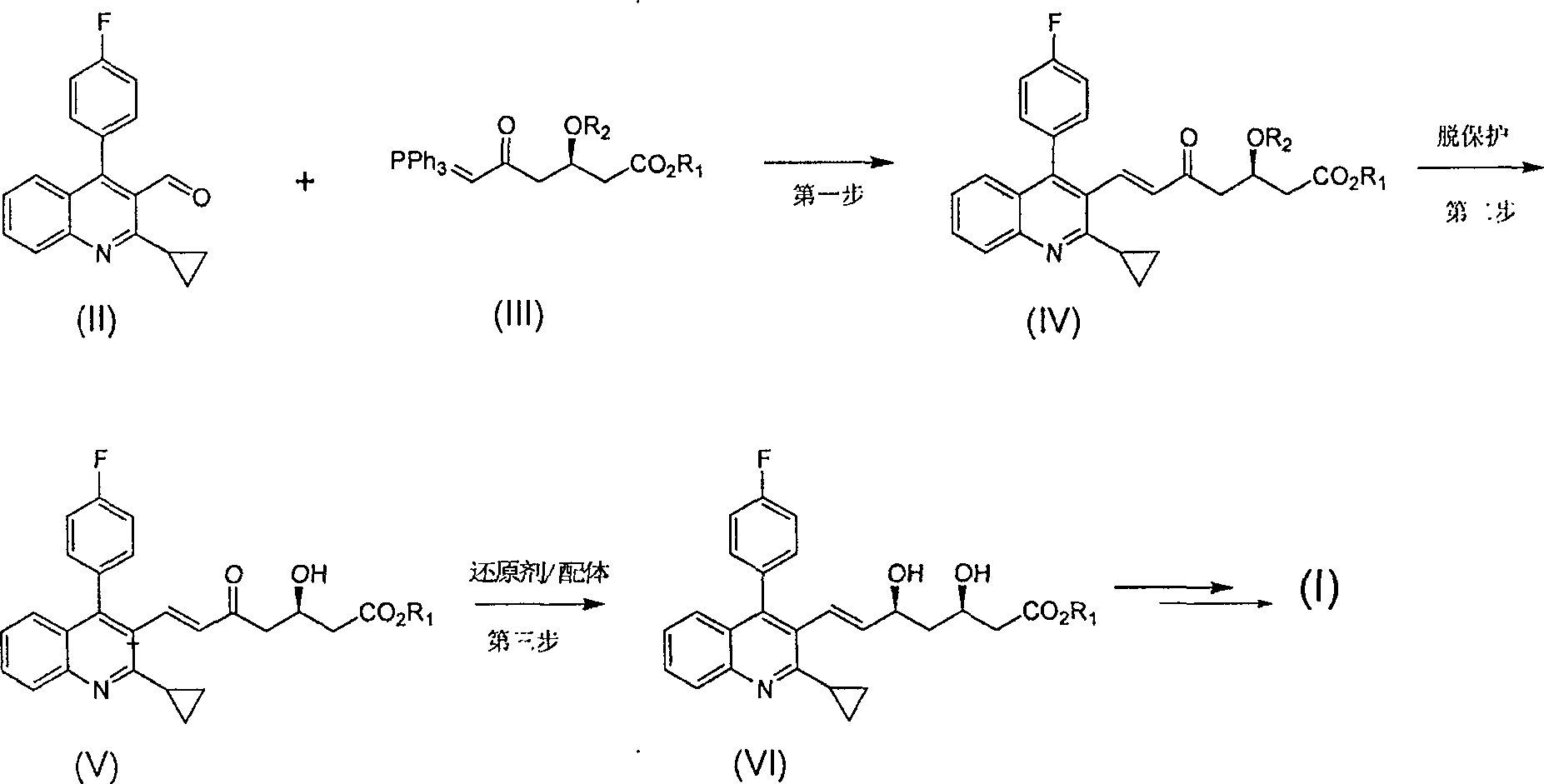
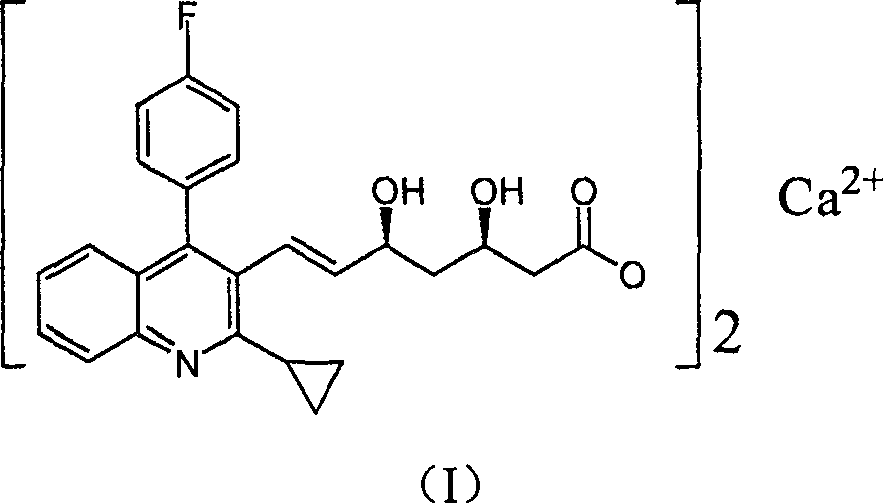
Method for preparing high optical purity pitavastatin calcium raw material drug
A technology of pitavastatin calcium and raw materials, which is applied in the field of preparation of cholesterol-lowering drugs, can solve problems such as low yield and difficult separation and purification, and achieve low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
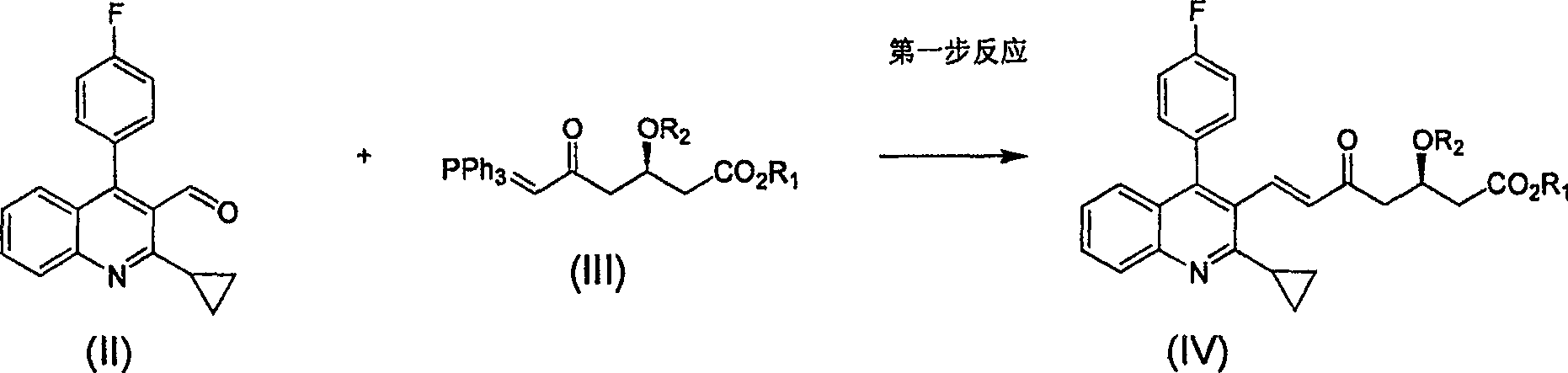
[0032] 1: (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline]-5-carbonyl-(3R)-3-(tert-butyldimethylsiloxane base)-6-heptenoic acid methyl ester preparation
[0033] Reaction formula:
[0034]
[0035] Steps
[0036] Put 27g of compound II and 62.5g (1.25eq) of phosphorus ylide 1 into a 1L single-port reaction flask, add 680ml of anhydrous acetonitrile, stir, heat to 70-80°C for 24 hours, and TLC monitors that the reaction is basically complete. The solvent was distilled off, and the residue was 45.7 g of a slurry separated by column. Yield 90%.
[0037] [α] D 25 : -8.29 (c, 1.2; MeOH))
[0038] HNMR (CDCl 3 )δ: 0.00(s, 3H), 0.05(s, 3H), 0.8(s, 9H), 1.10(q, 2H), 1.40(s, 2H), 2.30(m, 1H), 2.46(m, 2H ), 2.69(m, 2H), 4.57(p, 1H), 3.66(s, 3H), 6.3(d, 1H), 7.63(d, 1H), 7.1-8.0(8H)
[0039] MASS: (Base)548.7(M+1)
[0040] C 32 h 38 FNO 4 Si: cal.547.7
[0041] 2: Preparation of (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline]-5-carbonyl-(3R)-hydroxy-6-heptenoic ac...
Embodiment 2
[0082] 1: (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline]-5-carbonyl-(3R)-3-(tert-butyldimethylsiloxane ) Preparation of ethyl 6-heptenoate
[0083] Reaction formula:
[0084]
[0085] Steps
[0086] Put 18g of compound II and 66.01 (2.0eq) of phosphorus ylide 1 into a 1L single-port reaction flask, add 700ml of anhydrous THF, stir, heat to 60-70°C for 48 hours, and TLC monitors that the reaction is basically complete. The solvent was distilled off, and the residue was 27.4 g of slurry separated by column, but the yield decreased to 81%.
[0087] [α] D 25 : -8.10 (c, 1.1; MeOH).
Embodiment 3
[0089] 1: (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline]-5-carbonyl-(3R)-3-(tert-butyldimethylsiloxane ) Preparation of ethyl 6-heptenoate
[0090] Reaction formula:
[0091]
[0092] Steps
[0093]Put 18g of compound II and 59.4 (1.8eq) of phosphorus ylide 1 into a 1L single-port reaction flask, add 600ml of anhydrous toluene and stir, heat to 100°C for 12 hours, and TLC monitors that the reaction is basically complete. The solvent was distilled off, and the residue was 28.4 g of slurry separated by column, the yield was 85%.
[0094] [α] D 25 : -8.10 (c, 1.1; MeOH)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com