A preparation method of argatroban intermediates
A technology for argatroban and intermediates, which is applied in the field of preparing argatroban intermediates, can solve the problems of unsuitability for industrial production, many reaction by-products, and difficult separation, and achieve complete reaction, simple operation, and easy purification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
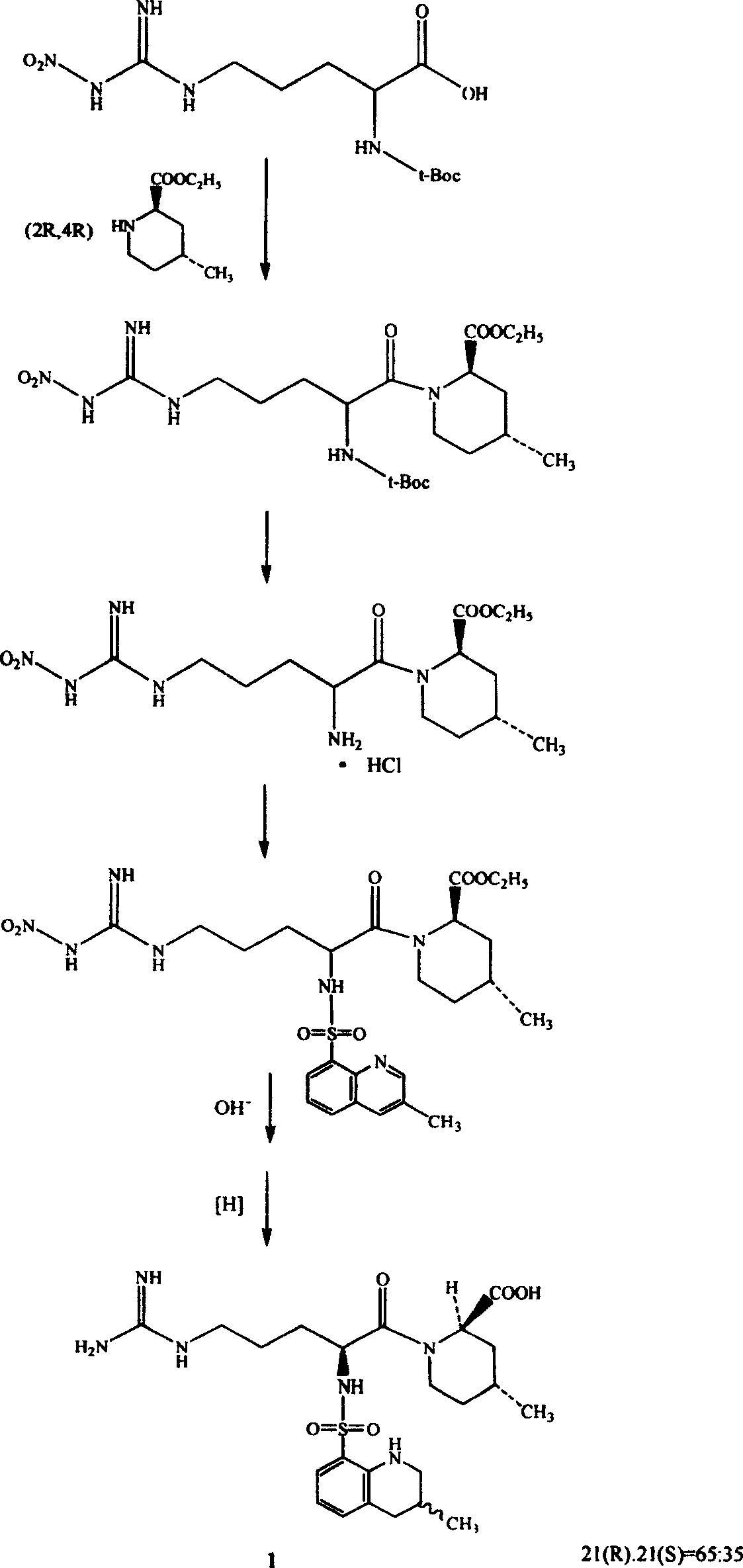
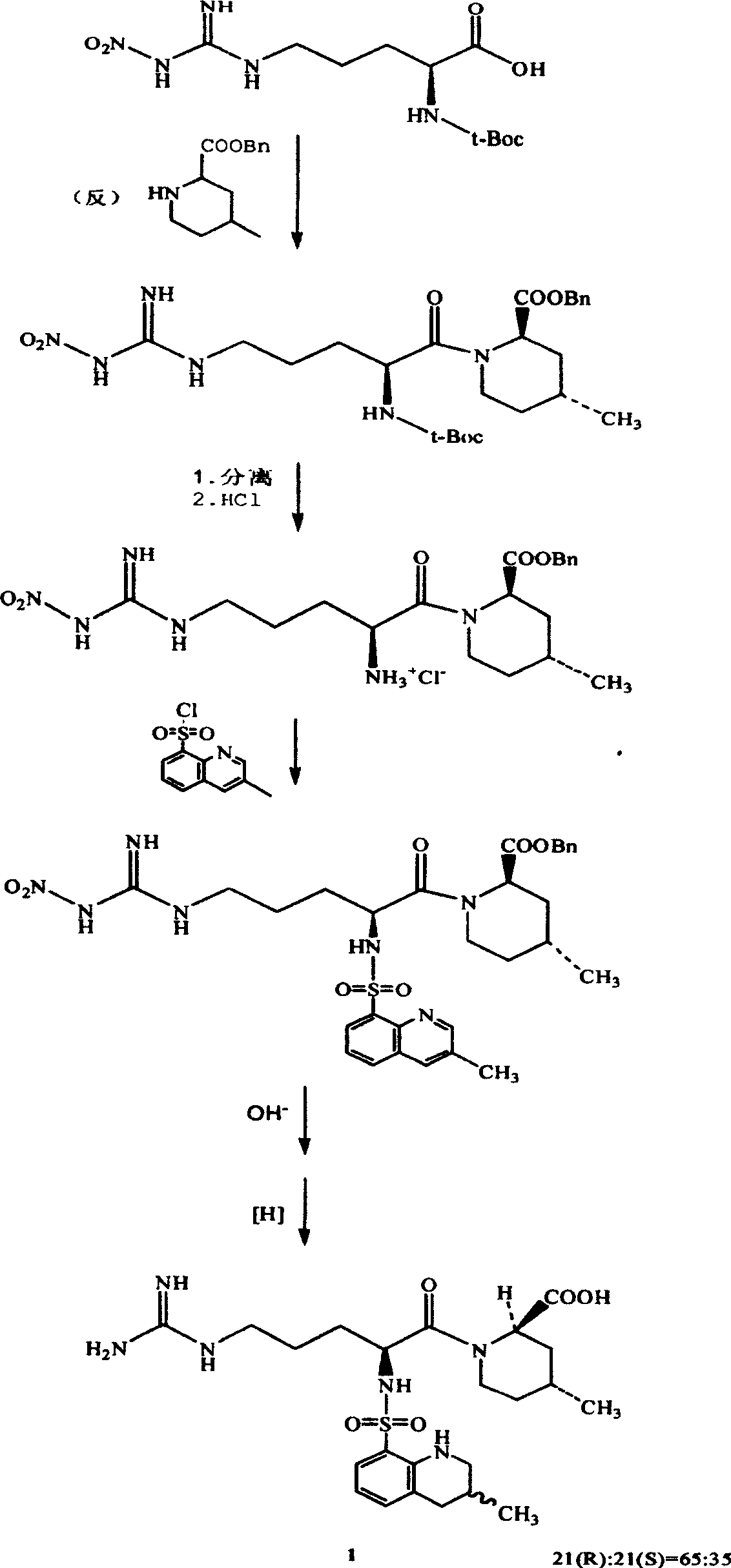
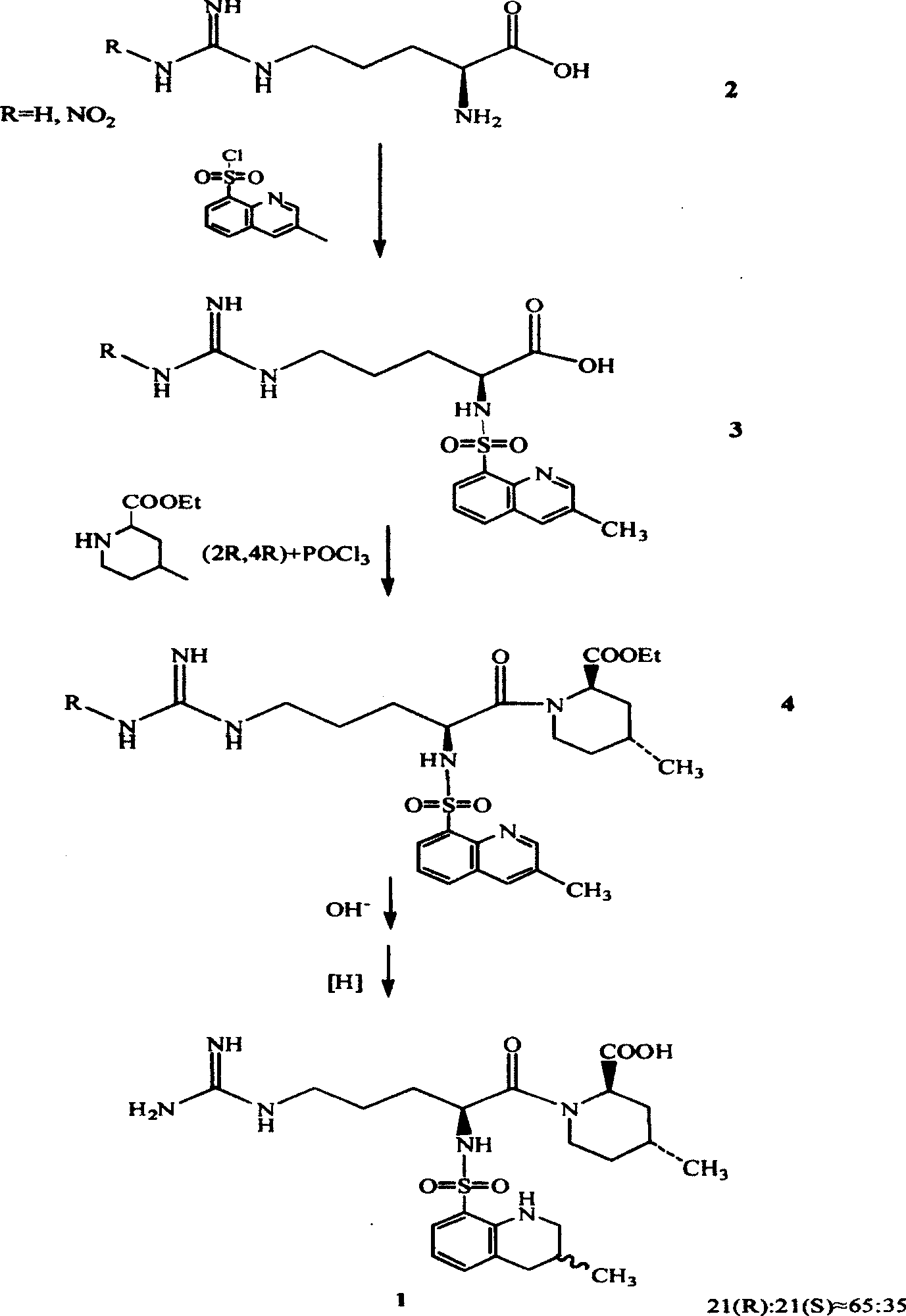
[0029] Example 1.N G - Preparation of nitro-L-arginine
[0030] Add 165 g of concentrated sulfuric acid into a 300 ml four-necked reaction flask, add 34.8 g (0.2 mol) of L-arginine with stirring, stir until transparent, add 20.0 g (0.4 mol) of ammonium nitrate, and stir at room temperature for 3 hours. After the reaction, the reaction solution was poured into 600 grams of ice water, neutralized with about 370 grams of ammonia (17%), controlled to pH 6.5, and a white solid was precipitated, filtered, washed with water, and recrystallized with 900 grams of water to obtain N G -Nitro-L-arginine 35.7 grams, content 98.5% (HPLC quantitative analysis method), yield 80.3%.
Embodiment 2
[0031] Example 2.N G —Nitro—N 2 - Preparation of tBoc-L-arginine
[0032] Add 100ml of water into a 300ml four-necked reaction flask, add 4.4 grams (1.1mol) of sodium hydroxide under stirring, cool after stirring and dissolving, add 20.0 grams (0.091mol) of NG -Nitro-L-arginine and 52 grams of tert-butanol, stirred for a while to obtain a transparent solution, added 25.9 grams (0.119mol) of di-tert-butyl carbonate at a liquid temperatureG —Nitro—N 2 —tBoc—L—arginine (HPLC quantitative analysis method), content 96.5%, yield 57.8%.
Embodiment 3
[0033] Embodiment 3.(2R, 4R)-1-[N G —Nitro—N 2 -(tert-butyloxycarbonyl)-L-arginine]-4-methyl-2-piperidinecarboxylate ethyl ester
[0034] In a 5000ml four-necked reaction flask, add 3000ml of dichloromethane, under stirring, add 112.8g (0.655mol) of (2R,4R)-4-methyl-2-piperidinecarboxylate ethyl ester, and dissolve it in about 0.5 hours. Under stirring at room temperature, add DCC134.8 g (0.655 mol), continue stirring for 0.5 hours, add N G —Nitro—N 2 —tBoc—L—209.0 g (0.655 mol) of arginine, stirred at room temperature for 2 hours, detected by TLC (solvent system is ethyl acetate), the raw materials basically disappeared, and the reaction ended. Add 600ml of water, stir for 0.5 hours, cool the reaction solution to 0°C, filter to remove the by-product urea, separate the filtrate, remove the water layer, wash the organic layer twice with 950ml of 10% sodium hydroxide solution, wash with 600ml of 10% citric acid The solution was washed to neutrality, then washed with 600 ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com