Bioderived amnion, composite bioderived amnion and its preparation method
An amniotic membrane and biological technology, applied in biochemical equipment and methods, microorganisms, tissue culture, etc., can solve the problems of high cost of trypsin treatment, ineffective removal of antigen components, etc., to shorten skin healing time, promote cell adhesion, The effect of preventing tissue adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
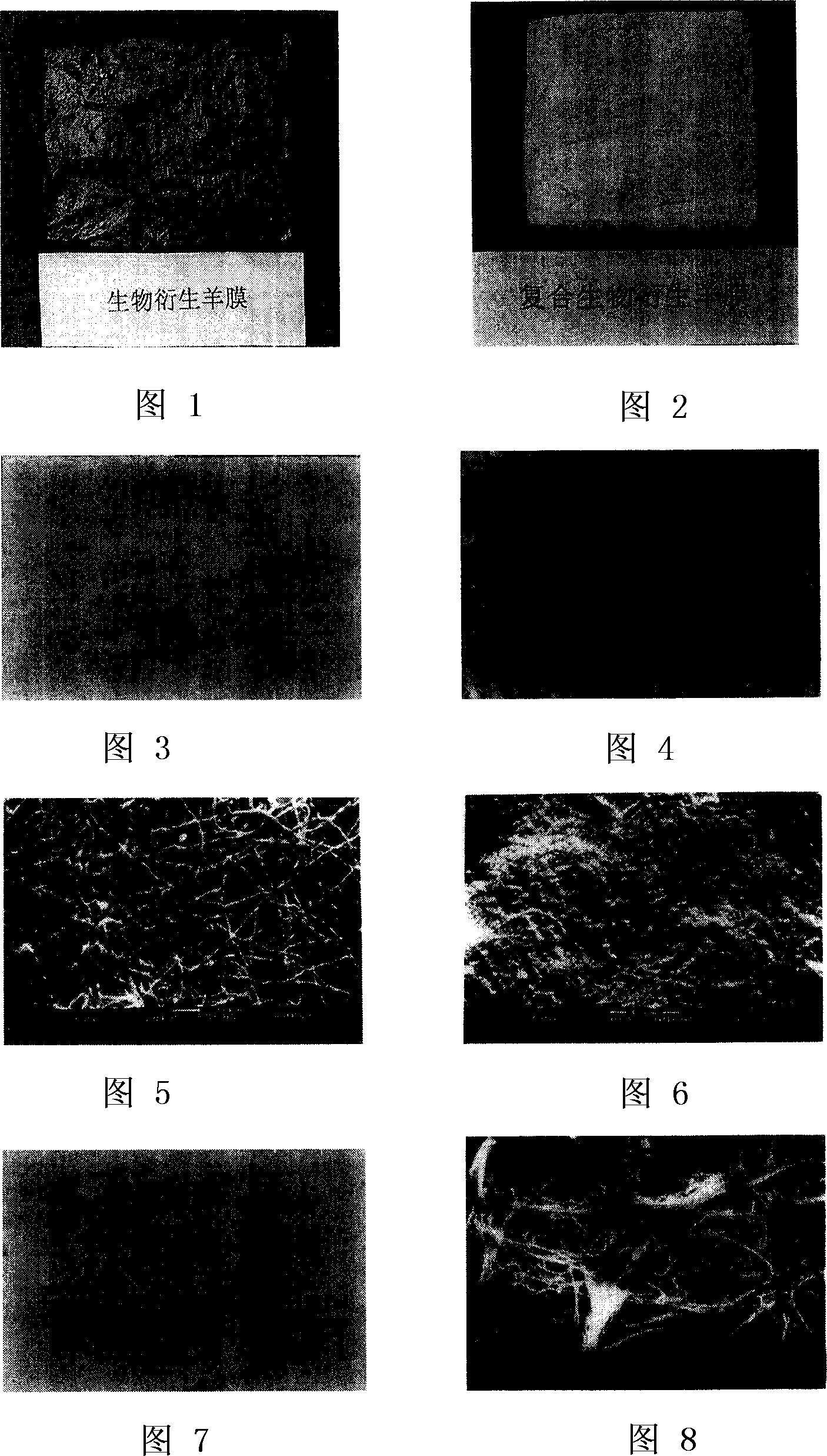
[0050] Example 1 Preparation of biologically derived amniotic membrane
[0051] Take fresh healthy human amniotic membrane, rinse with normal saline for 3 times, degrease with chloroform / methanol (1:1, v / v) until the supernatant is clear, cross-link with 0.25% glutaraldehyde for 10 minutes, rinse with normal saline for 3 times, each time for 10 minutes; Treat with 0.5% w / v SDS (sodium dodecylsulfonate) for 4 hours, rinse with normal saline 3 times, 10 minutes each time; digest with 0.25% w / v trypsin for 8 hours, rinse with normal saline 3 times, 10 minutes each time , freeze-dried, subpackaged, sealed, and sterilized with ethylene oxide.
Embodiment 2
[0052] Example 2 Preparation of Composite Biologically Derived Amniotic Membrane
[0053] Collagen is extracted from human tendon by acid extraction, and 0.3% acetic acid solution is used to prepare a 0.5% collagen solution. The biologically derived amniotic membrane prepared in Example 1 that has not been lyophilized after treatment is covered in stainless steel containers of different specifications (such as 40x40x8mm ) bottom, add collagen solution according to the required material thickness, freeze at -80°C for half an hour, freeze-dry in a freeze dryer for 24 hours, cross-link in 0.25% glutaraldehyde solution at 4°C for 12 hours, fully rinse with pure water, wash The residual glutaraldehyde was removed, and then freeze-dried to obtain the composite bio-derived amniotic membrane. Pack and seal, and sterilize by ethylene oxide or gamma ray irradiation.
Embodiment 3
[0054] Physical properties of embodiment 3 biologically derived amniotic membrane and composite biologically derived amniotic membrane
[0055] The physical properties of the biologically derived amniotic membrane prepared in Example 1 and the composite biologically derived amniotic membrane prepared in Example 2 are as follows:
[0056] Bioderived amnion is a colorless film that is easily rehydrated and becomes transparent after rehydration (Figure 1). Composite bio-derived amnion is a yellow spongy film whose thickness can be determined according to needs (Figure 2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com