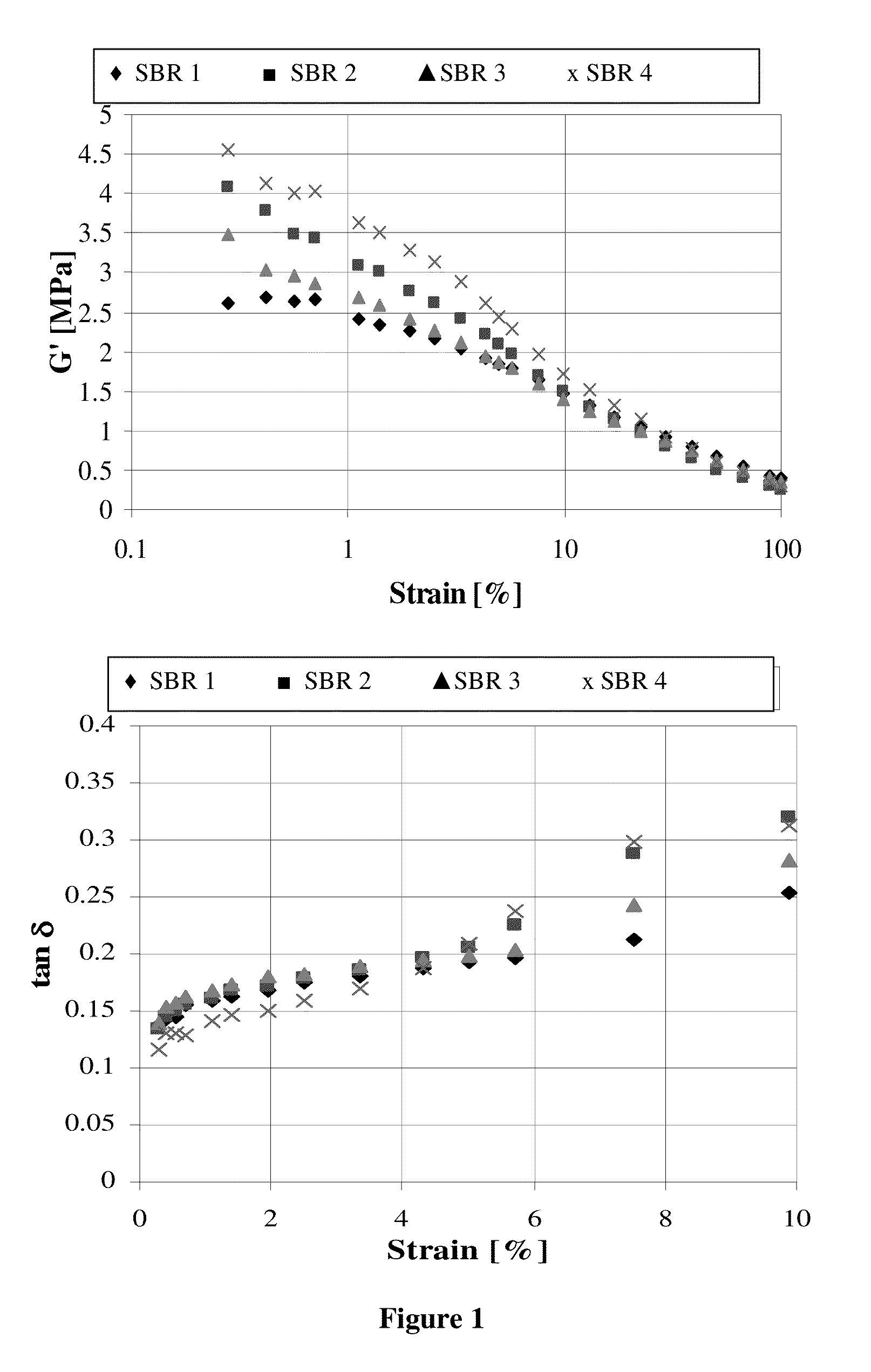
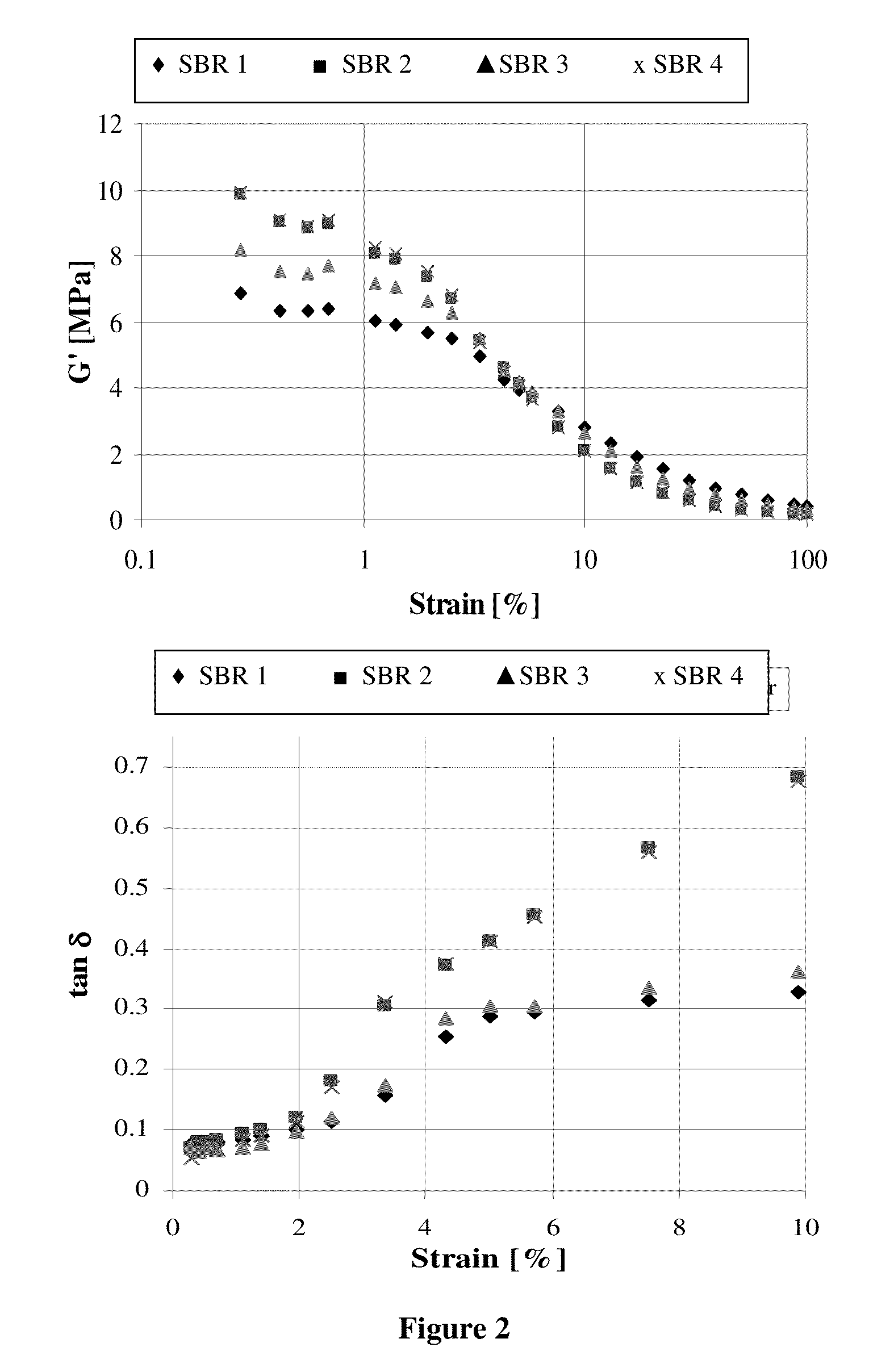
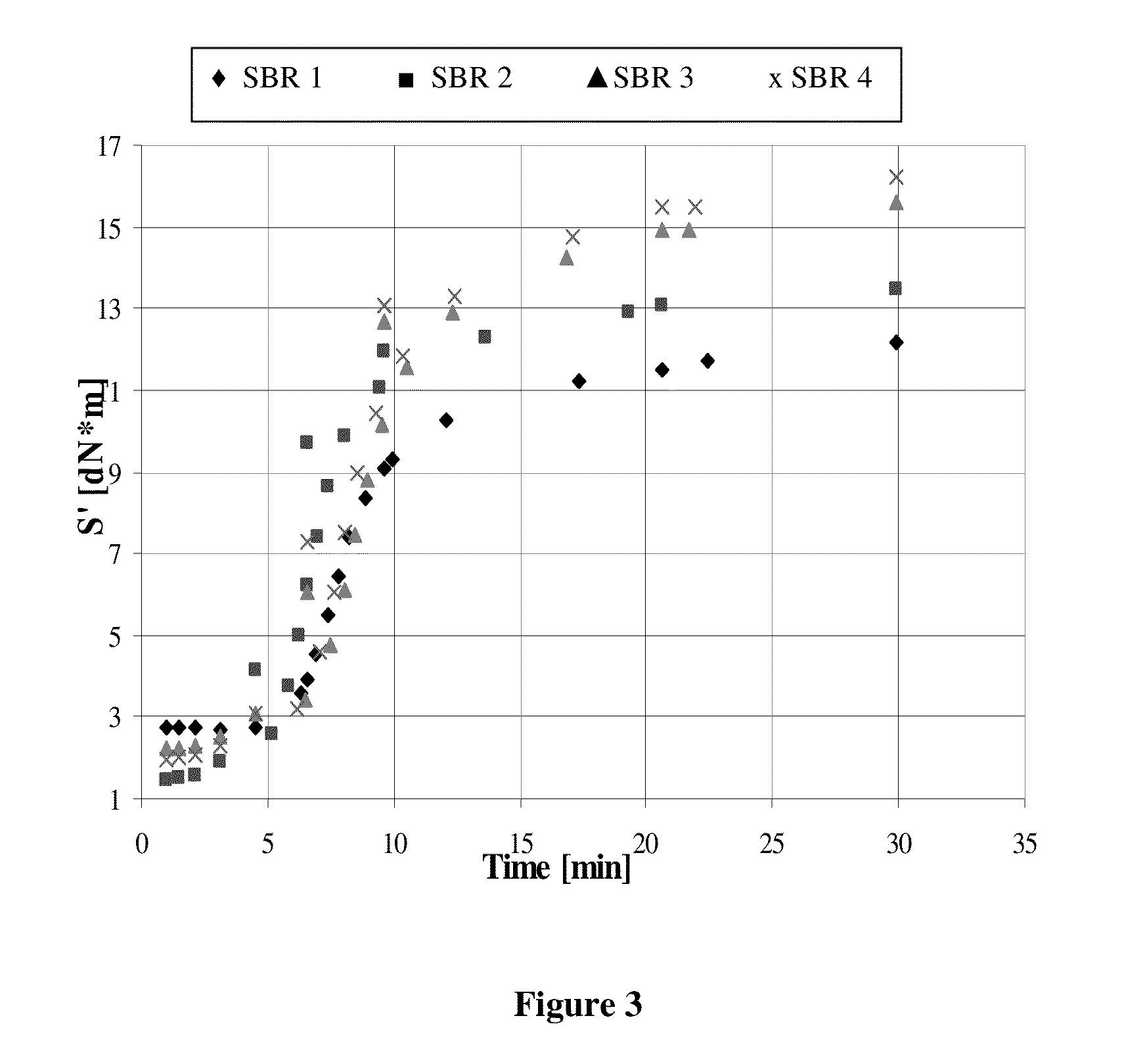
Functionalized polymer, rubber composition and pneumatic tire
a technology of functionalized polymers and rubber compositions, applied in the direction of special tyres, transportation and packaging, tyre parts, etc., can solve the problems of low polymer hysteresis and provide a lower level of tire rolling resistance, so as to improve the affinity of fillers, improve the interaction of polymer/filler, and reduce the cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of S-Benzyl S′-trimethoxysilylpropyltrithiocarbonate
[0061]
[0062]To a stirred solution of 3-(mercaptopropyl)trimethoxysilane (30 mmol) in 50 mL of anhydrous methanol was added dropwise a solution of sodium methoxide in methanol (25 wt % in methanol, 30 mmol) under nitrogen. After stirring for 30 min, carbon disulfide (40 mmol) was added dropwise to the solution, and the mixture was stirred at ambient temperature for 3 h. To the yellow solution was added benzyl bromide (30 mmol) and the mixture was stirred for 3.5 h under nitrogen. The mixture was concentrated, diluted with dichloromethane, filtered off, concentrated under reduced pressure and dried overnight in Schlenk line. The purity of synthesized compound was characterized via 1H-NMR (400 MHz).
example 2
Synthesis of S-Benzyl S′-triethoxysilylpropyltrithiocarbonate
[0063]
[0064]To a stirred solution of 3-(mercaptopropyl)triethoxysilane (30 mmol) in 50 mL of anhydrous ethanol was added dropwise a solution of sodium ethoxide in ethanol (21 wt % in ethanol, 30 mmol) under nitrogen. After stirring for 30 min, carbon disulfide (40 mmol) was added dropwise to the solution, and the mixture was stirred at ambient temperature for 3 h. To the yellow solution was added benzyl bromide (30 mmol) and the mixture was stirred for 3.5 h under nitrogen. The mixture was concentrated, diluted with dichloromethane, filtered off, concentrated under reduced pressure and dried overnight in Schlenk line. The purity of synthesized compound was characterized via 1H-NMR (400 MHz).
example 3
Synthesis of Silane, trimethoxy[3-(phenylmethyl)thio]propyl]
[0065]
[0066]To a stirred solution of 3-(mercaptopropyl)trimethoxysilane (30 mmol) in 50 mL of anhydrous methanol was added dropwise a solution of sodium methoxide in methanol (25 wt % in methanol, 30 mmol) under nitrogen. After stirring for 30 min, benzyl bromide (30 mmol) was added dropwise to the solution, and the mixture was stirred at ambient temperature overnight. The mixture was concentrated, diluted with dichloromethane, filtered off, concentrated under reduced pressure and dried overnight in Schlenck line. The synthesized compound was characterized via 1H-NMR (400 MHz).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com