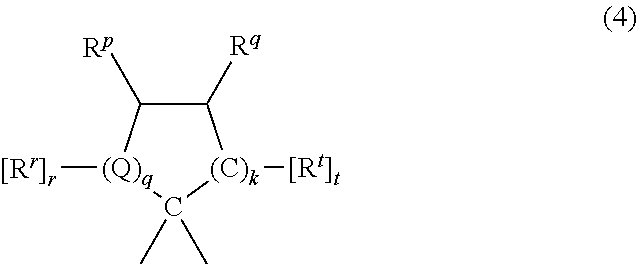
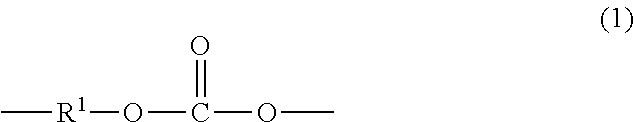Manufacture of dihydroxy aromatic compounds by alcoholysis of polycarbonate-containing compositions
a technology of alcoholysis and polycarbonate, which is applied in the preparation of organic carbonates, organic compound preparations, organic chemistry, etc., can solve the problems of difficult depolymerization of polycarbonates in wastes, significant bulk waste disposal problems, and inability to biodegrade polycarbonates, etc., to achieve the effect of reducing the color of crude dihydroxy aromatic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0072]43 g of standard BPA was dissolved in 300 ml of methanol to form a clear solution. After 0.2 g of Ti-isopropoxide catalyst was added the solution turned yellow. The solution had an APHA value of 2112 (sample A). A 100 ml sample of this yellow solution was taken, and 50 ml of 15% NaOH solution was added at room temperature. There was a reduction in the yellow color of the solution. The solution was neutralized with 50% HCl solution until the precipitation of BPA was complete. The APHA of the obtained BPA was measured to be 612.
example 2
[0073]43 g of standard BPA was dissolved in 300 ml of methanol to form a clear solution. Then 0.2 g of Ti-isopropoxide catalyst was added to the solution. The solution turned yellow. A 100 ml sample of this yellow solution was taken, and 50 ml of 5% HCl solution was added and heated for 30 min at 100° C. The solution turned colorless, and the mixture was brought to room temperature. Water was added until precipitation was complete. The precipitated BPA was filtered. The APHA value of the obtained BPA was measured to be 56. Prior to acid treatment, the BPA sample (sample A) had an APHA of 2112.
examples 3-14
[0074]A series of experiments were carried out to reduce the color of crude BPA obtained from methanolysis of polycarbonate-containing compositions. The color values of the samples on the American Public Health Association color index (referred to as “APHA values”) are listed in Table 2. These experiments showed that BPA purity could be improved by toluene crystallization in the presence of an acid. Recrystallization of crude BPA with toluene enhances the purity from 79% to 99.7%. The results of various acids on the color by toluene crystallization have been tabulated along with the APHA value on Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com