Preparation of catalyst and use for high yield conversion of methane to ethylene
a technology of methane and catalyst, which is applied in the field of synthesis of higher hydrocarbons from methane, to achieve the effect of enhancing conversion characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
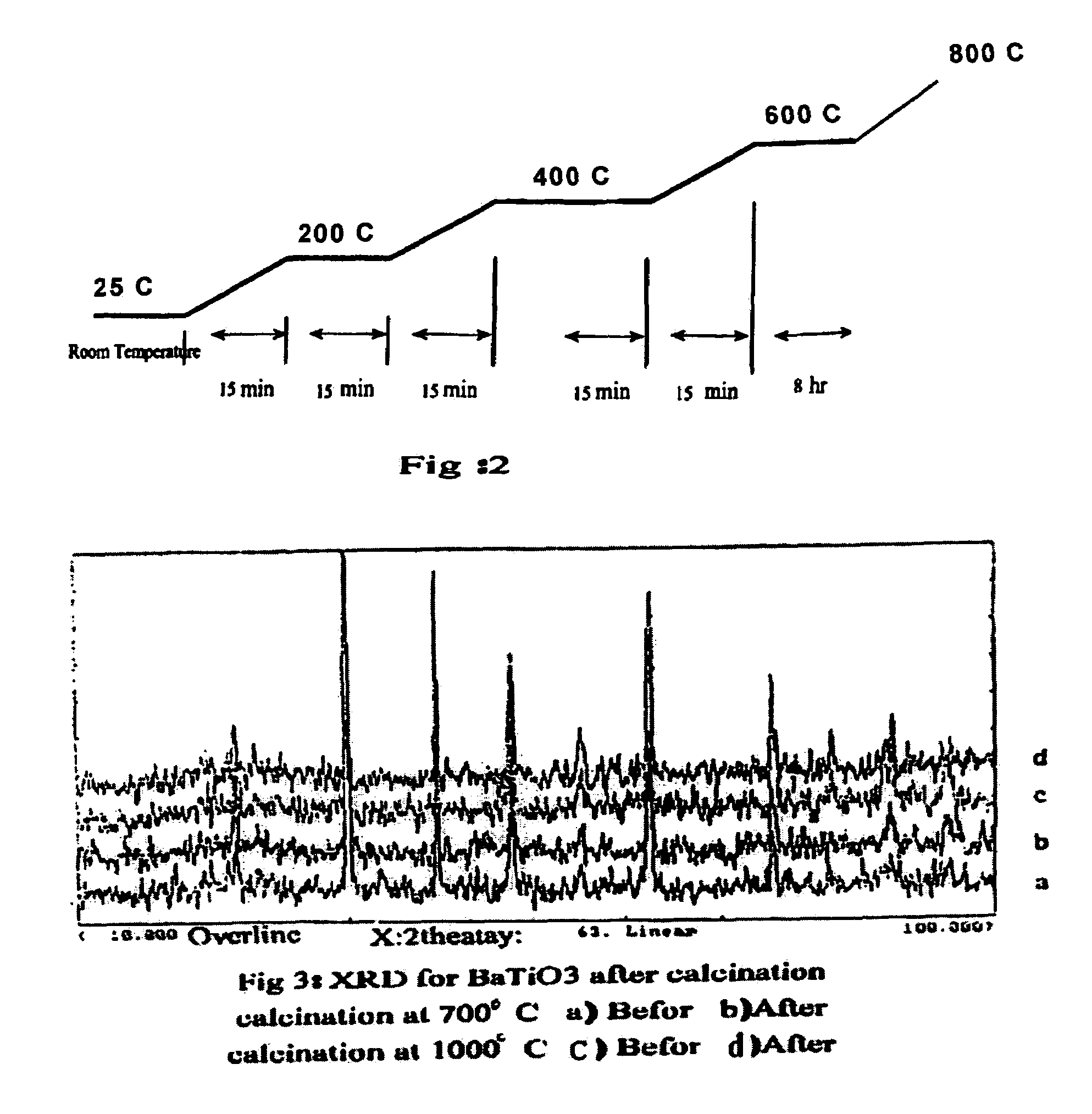
[0046]A variety of catalysts were prepared using the sol-sol methodology of the present invention. Different alkali earth metals were applied for use in a direct conversion reaction for the production of ethylene from methane. The catalysts were prepared employing the basic perovskite crystal formula, ABTiO3, so as to determine the optimum perovskite catalyst which is capable of reproducibly producing the greatest yield of ethylene. Combinations were tried including A=tin (Sn) and wherein B was selected from=Ca,Sr,Ba or combinations thereof.
[0047]Evaluations of this formulation were undertaken in the range of Ba ((1-2 moles−x)+TiO2 ((1 mole))+SnCl2(x) where x ranges between 0.09 and 0.1 moles, or 0.95 moles Barium per mole of TiO2 to 0.05 moles SnCl2.
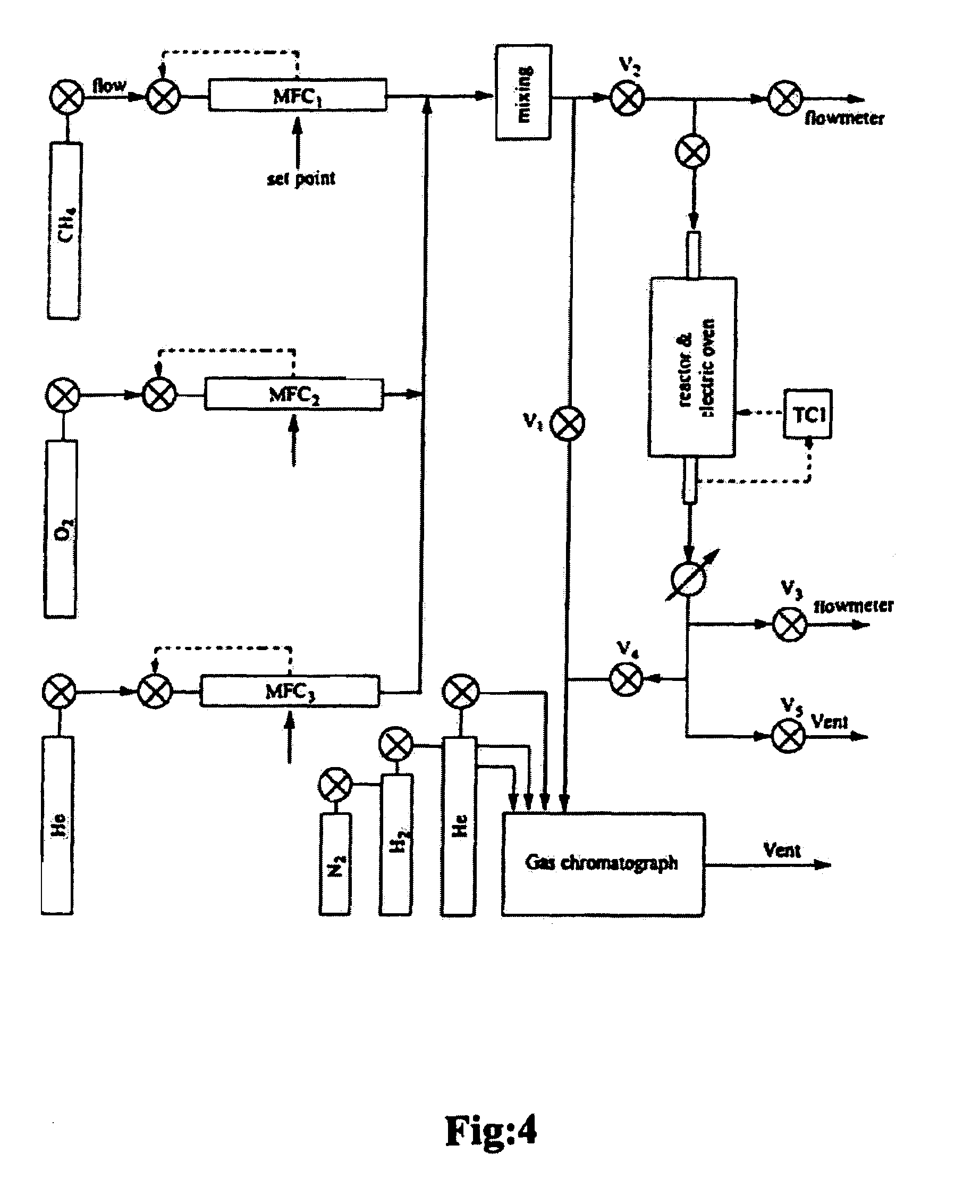
[0048]With reference to FIG. 4, the reactor experiments were carried out in a flow fixed-bed quartz reactor having a diameter of 10 mm at a temperature ranging from between about 500° C.-about 1000° C., but ...
example 2
Pilot Scale Oxidative Coupling of Methane: Effect of Halogen Addition
[0055]In this example, and in Example 3, OCM was performed using a pilot reactor with approximately 30.5 grams of a catalyst prepared from a mixture of Barium carbonate, Tin chloride and Titanium oxide, combined in a ratio of 39.3 grams; 11.4 grams; 19.2 grams. The resulting catalyst was then pelletized under 5.5 tons / cm3 and crushed into particles of between 1.96-3.96 mm diameter.
[0056]A model SS316 reactor tube having dimensions of 25 mm (O.D., 21 mm I.D.) by 490 mm in length was fitted with a quartz liner, and quartz packing, and the catalyst packed towards the middle of the reactor tube without dilution, as shown in FIG. 7 (illustrating top gas feed). The effects of both bottom and top gas feed injection were evaluated; the best results (Table 2) were achieved using bottom gas feed. The top and bottom portions of the reactor were packed with quartz particles of diameter ranging from 1.98-3.96 mm.
[0057]In this e...
example 3
[0064]For this example, the same catalyst as used in Example 2 was employed, but not diluted; no halogen was added to the reactor.
[0065]For this example, the parameters were as follows:[0066]Methane / Oxygen / Nitrogen molar ratio of inlet gas: CH4:O2:N2 was 2:1:8.[0067]GHSV=5123;[0068]WHSVCI=0.44;[0069]Quartz-lined reactor, no CCl4 added.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com