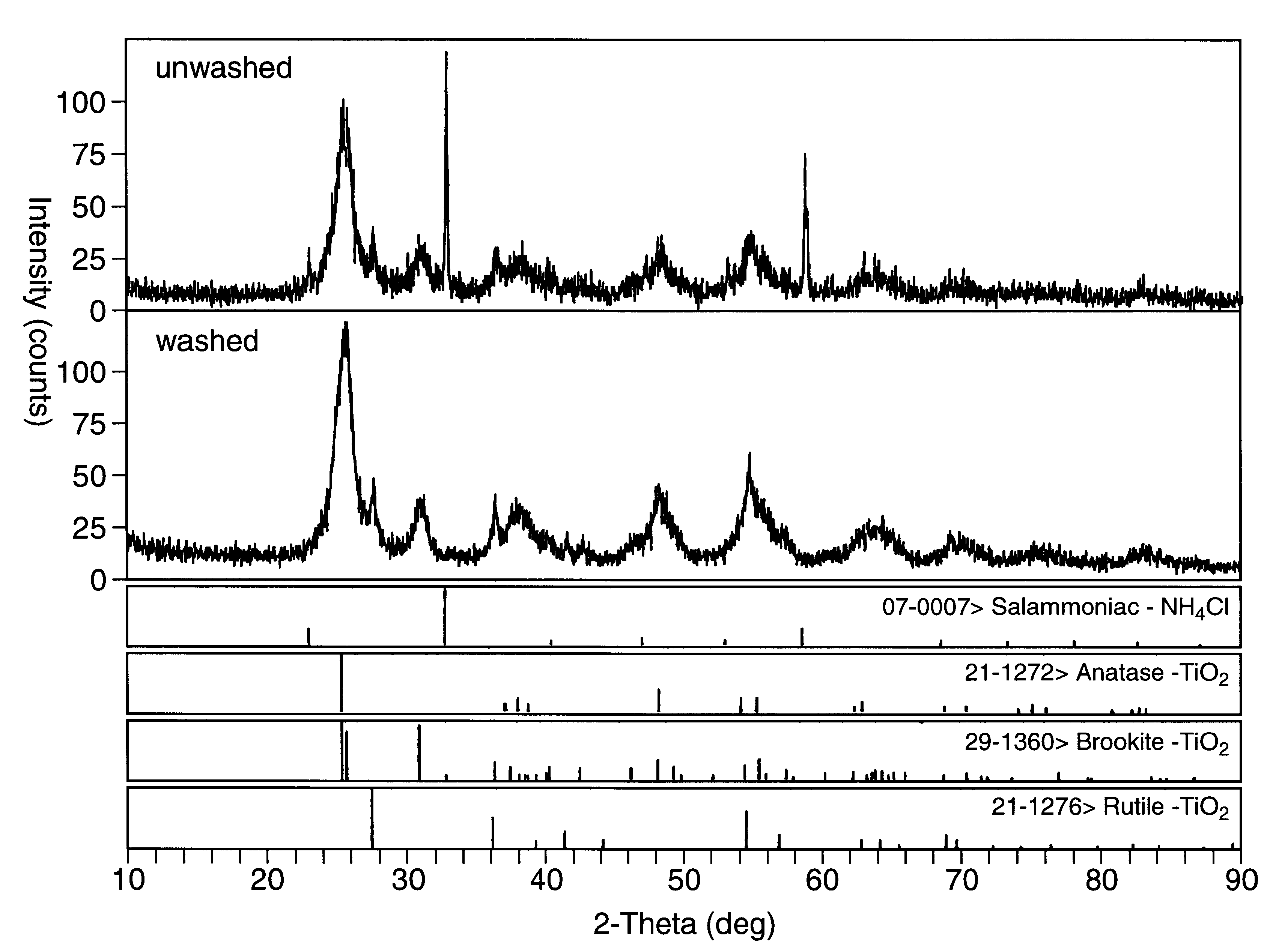
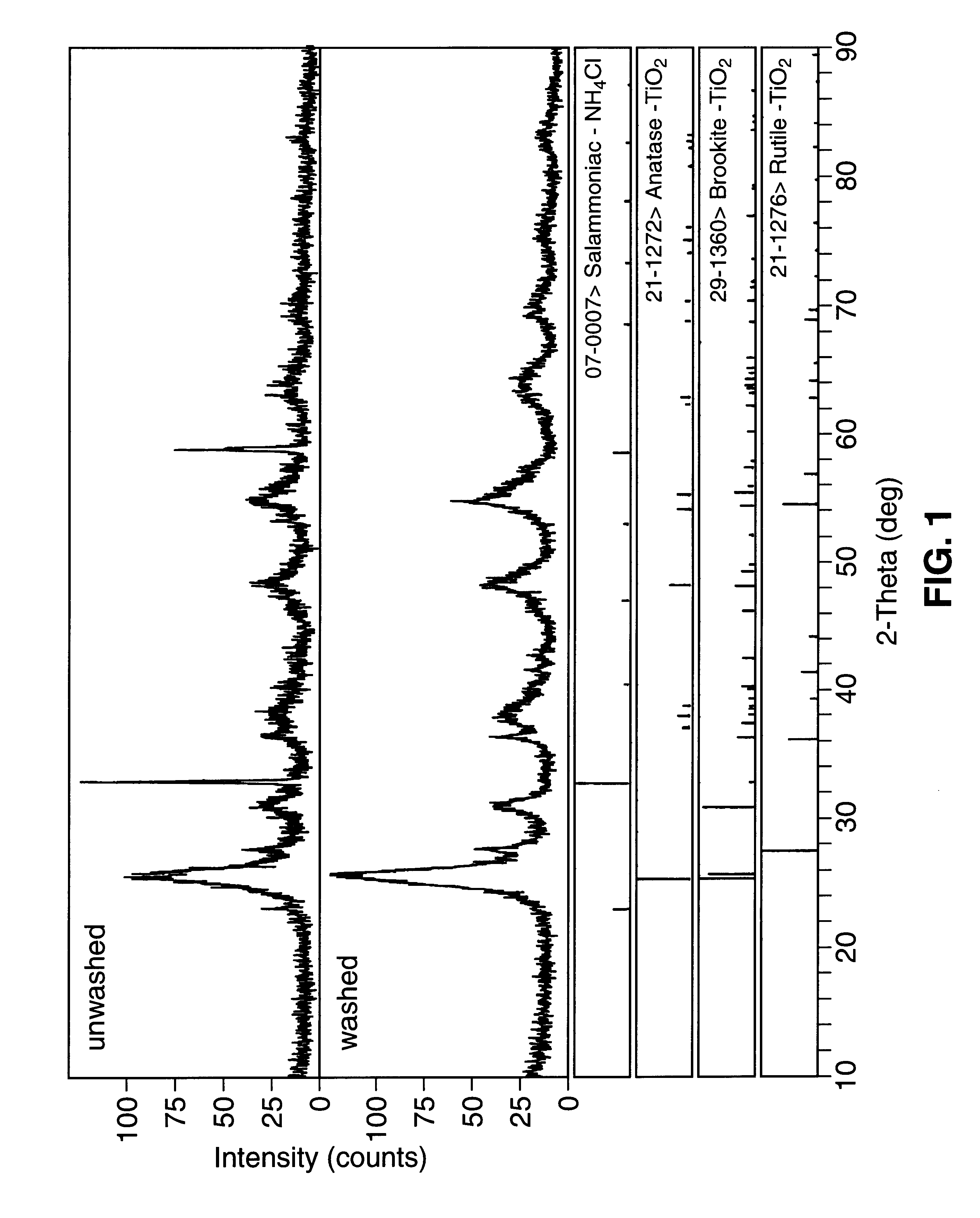
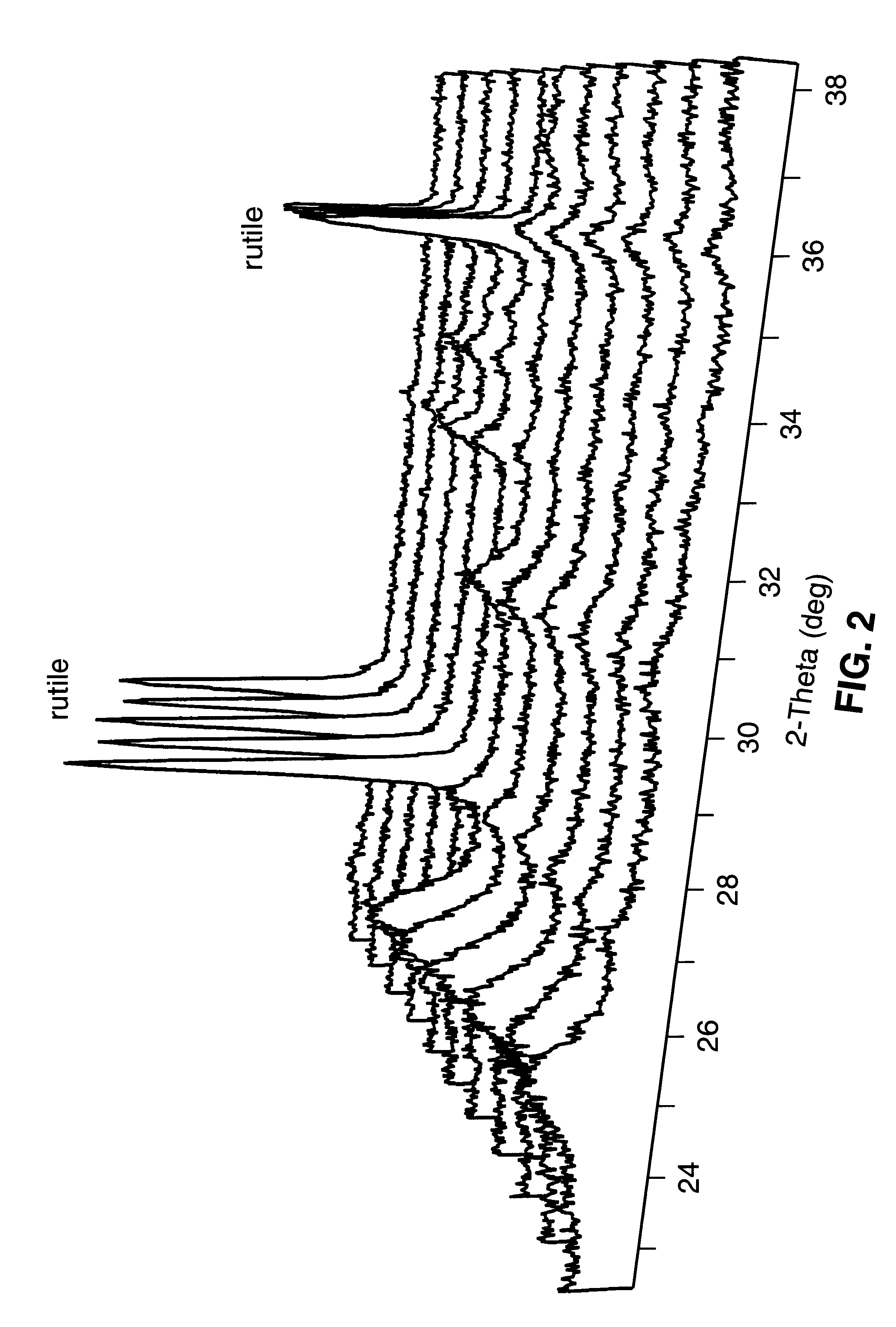
Methods for producing monodispersed particles of barium titanate
a technology of monodispersed particles and barium titanate, which is applied in the direction of titanium compounds, chemistry apparatuses and processes, zirconium compounds, etc., can solve the problems of inadequate existing ball milling operations, poor electrical property optimization and reproducibility, and insufficient microstructure for microelectronic applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Preparation of Starting Solutions
Titanium tetrachloride liquid (99.6% TiCl.sub.4 Alfa Aesar, Ward Hill, Mass.) (5.55 mL) was slowly added to icecold aqueous HCI solution (2.85 mL 1.0 N HCl in .about.20 mL distilled water), which was constantly stirred in a 50 mL volumetric flask. The acidity is needed to minimize the explosive generation of orthotitanic acid (Ti(OH).sub.4) and thus the uncontrolled precipitation during the dissolution TiCl.sub.4 in the aqueous solution. The final concentration of titanium in the prepared TiCl.sub.4 stock solution was 1.0 M. The solution became somewhat turbid during the dissolution step but gradually cleared up within approximately 30 minutes, while the temperature of the solution rose to room temperature (.about.23.degree. C.). This stock was freshly prepared for each set of experiments and kept overnight before use. The stearic dispersant, hydroxypropylcellulose (HPC), was used as a steric dispersing agent that adsorb on the surfaces of particles ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com