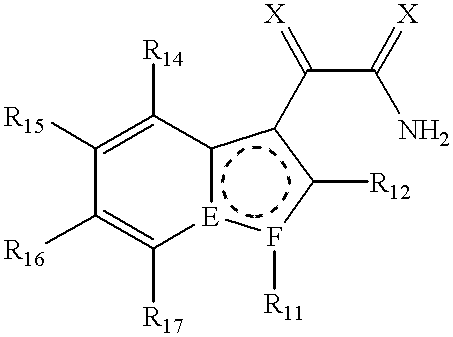
Method for the treatment of stroke using N-heterocyclic glyoxlyamide compounds
a technology of n-heterocyclic glyoxylamide and compound, which is applied in the field of stroke treatment using n-heterocyclic glyoxylamide compound, can solve the problems of reduced blood flow, death of brain tissue, death and disablement, etc., and achieve the effect of reducing neuronal cell death and minimizing the dose of n-heterocyclic glyoxylamide compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of [[3-(2-Amino-1,2-dioxoethyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy]acetic acid, a compound represented by the formula: ##STR24##
Part A. Preparation of 2-Ethyl-4-methoxy-1H-indole.
A solution of 140 mL (0.18 mol) of 1.3M sec-butyl lithium in cyclohexane was added slowly to N-tert-butoxycarbonyl-3-methoxy-2-methylaniline (21.3 g, 0.09 mol) in 250 mL of THF keeping the temperature below -40.degree. C. with a dry ice-ethanol bath. The bath was removed and the temperature allowed to rise to 0.degree. C. and then the bath replaced. After the temperature had cooled to -60.degree. C., 18.5 g (0.18 mol) of N-methoxy-N-methylpropanamide in an equal volume of THF was added dropwise. The reaction mixture was stirred 5 minutes, the cooling bath removed and stirred an additional 18 hours. It was then poured into a mixture of 300 mL of ether and 400 mL of 0.5N HCl. The organic layer was separated, washed with water, brine, dried over MgSO.sub.4, and concentrated at reduced press...
example 2
Part A: Preparation of Ethyl 3-benzyloxy-2-pyridineacetate 24
60% Sodium hydride (2.69 g, 66.2 mmol) was added in small portions to a solution of ethyl 3-hydroxy-2-pyridineacetate (23, 12.0 g, 66.2 m mol) (N. Desideri, F. Manna, M. L. Stein, G. Bile, W. Filippeelli, and E. Marmo. Eur. J. Med. Chem. Chim. Ther., 18, 295 (1983)) in dimethylformamide (220 ml) at 0.degree. C. The mixture was stirred at 0.degree. C. for 50 min. Benzyl chloride (8.4 ml, 72.8 mmol) was added dropwise to the mixture, which was stirred overnight. Ethyl acetate was added. The mixture was washed with 5% aqueous sodium hydrogencarbonate and water and dried over Na.sub.2 SO.sub.4. After removing the solvent at reduced pressure, the residue was chromatographed on silica gel eluting with AcOEt:toluene (1:19 to 1:1) to give 16.17 g (90.0% yield) of the titled compound as an oil.
IR .nu..sub.max (film) 1736, 1446, 1278 cm.sup.-1. .sup.1 H NMR (CDCl.sub.3) .delta. 1.21 (3H, t, J=7.2 Hz), 3.93 (2H, s), 4.14 (2H, q, J=7....
example 3
This example illustrates the effect of [[3-(2-Amino-1,2-dioxoethyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy]acetic acid (the compound prepared by Example 1, hereinafter called "Ex-1") on cerebral infarction in a rat focal stroke model.
Experimental protocol:
Wistar male rats weighing 240-260 g were used. The body temperature of the animals was maintained at 37.5.degree. C. with a heating pad during the operation. Anesthesia was induced with 3% halothane in 30% oxygen and maintained with 1-1.5% halothane in 30% oxygen. A catheter for the administration of rose bengal and Ex-1 was placed in the femoral vein. A subtemporal craniotomy was performed using a dental drill under an operating microscope to open a 3-mm diameter circular bone window, through which photo-irradiation with green light (wave length, 540 nm) was achieved by using a xenon lamp (Umemura et al. Stroke 24, 1077-1082, 1993). The head of optic fiber with 3-mm-diameter was placed on the window in the skull base, and rose...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com