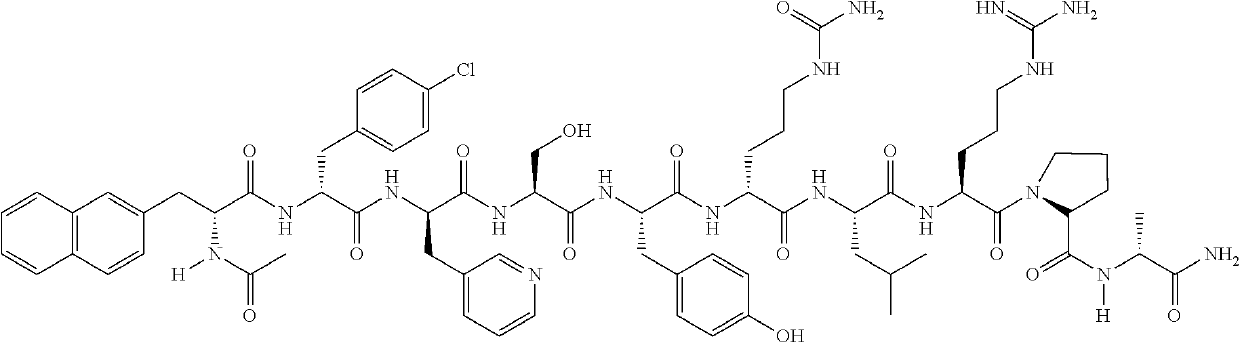
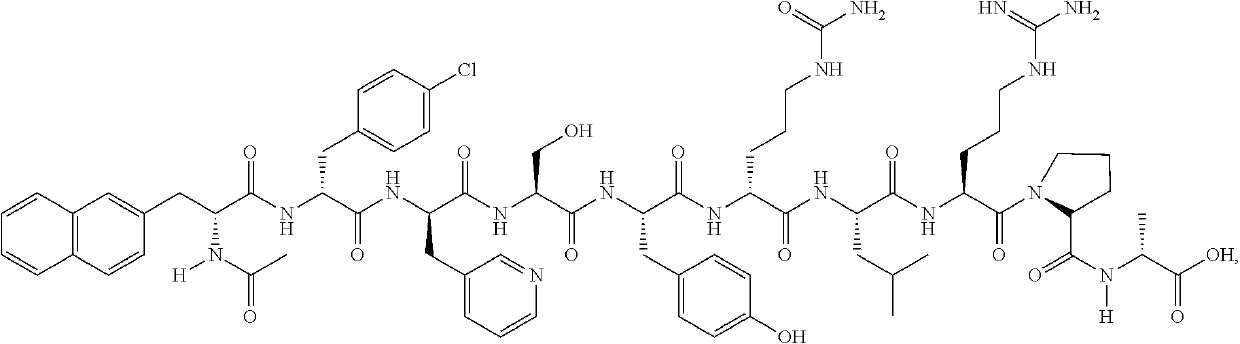
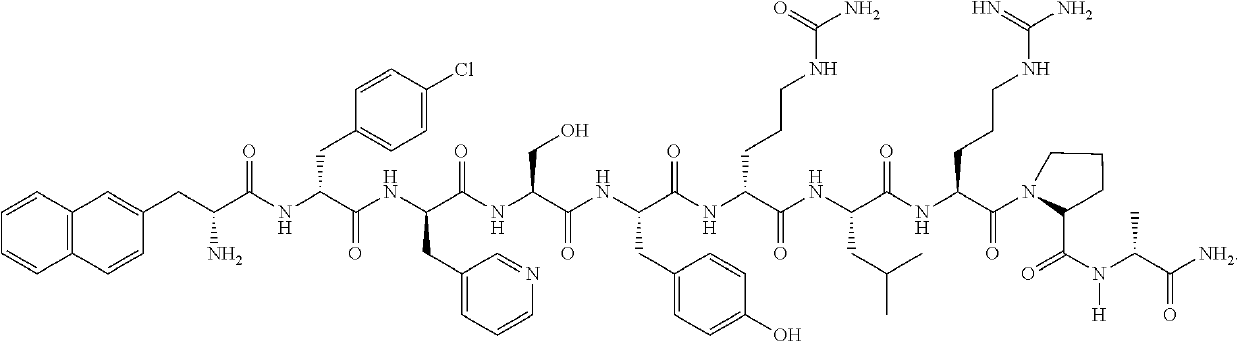
A stable parenteral dosage form of cetrorelix acetate
a parenteral and acetate technology, applied in the field of stable parenteral dosage form of cetrorelix acetate, can solve the problems of increased turbidity or cloudiness of the solution, easy chemical degradation of the solution, and expensive and time-consuming process of aqueous solution of peptides such as cetrorelix
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1a
Identification of the Degradation Product
[0100]In order to investigate the degradation of cetrorelix, peptide related substances of cetrorelix were prepared by the known technique of solid phase peptide synthesis. The synthesis involved coupling of one amino acid at a time sequentially starting from c-terminal amino acid on a resin. The synthesis of the peptide chain was carried out using the Fluorenylmethyloxycarboyl (Fmoc) / tButyl (Fmoc / tBu) with N,N′-diisopropyl carbodiimide (DIPC) as the coupling reagent. The Fmoc groups were removed via treatment with 20% piperidine in dimethylformamide. The peptide formed on resin was finally cleaved using trifluoroacetic acid to obtain related substances which were further purified by reverse phase high performance liquid chromatography (RP-HPLC) on a C18 Silica column using a gradient of acetonitrile / water containing 0.1% trifluoroacetic acid. The purified peptide related substances were lyophilized to obtain pure solid form. The structure of...
example 1b
[0106]Cetrorelix and the identified impurities namely, Impurity A, Impurity B, Impurity D and Impurity F from the aqueous solution samples were separated on a reverse phase (C-18) column using gradient technique (Column: X-Select CHS C18, (150×4.6) mm, 2.5p. (by Waters, Ireland, Part No: 186006729), detected and quantified by Ultraviolet spectroscopy at 225 nm wavelength. The mobile phase was run at a flow rate of 0.7 ml / min and 1.0 ml / min. The run time of the chromatogram was 150 minutes.
Mobile Phase Details:
[0107]Mobile Phase A: A mixture of buffer solution as below, with acetonitrile and tetrahydrofuran in the ratio of (700:280:20), degassed by sonication.
Mobile Phase B: A mixture of buffer solution as below, with acetonitrile and tetrahydrofuran in the ratio of (500:480:20), degassed by sonication.
Buffer: 2.5 g of Ammonium dihydrogen orthophsphate and 0.75 g of 1-Octane sulphonic acid sodium salt in 1000 ml water with pH adjusted to 8.0±0.05 using triethylamine.
Diluent: A mixtur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com