Method of treating hidradentitis suppurativa with il-17 antagonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
dies of Anti-IL-17 Antibodies in Treating HS
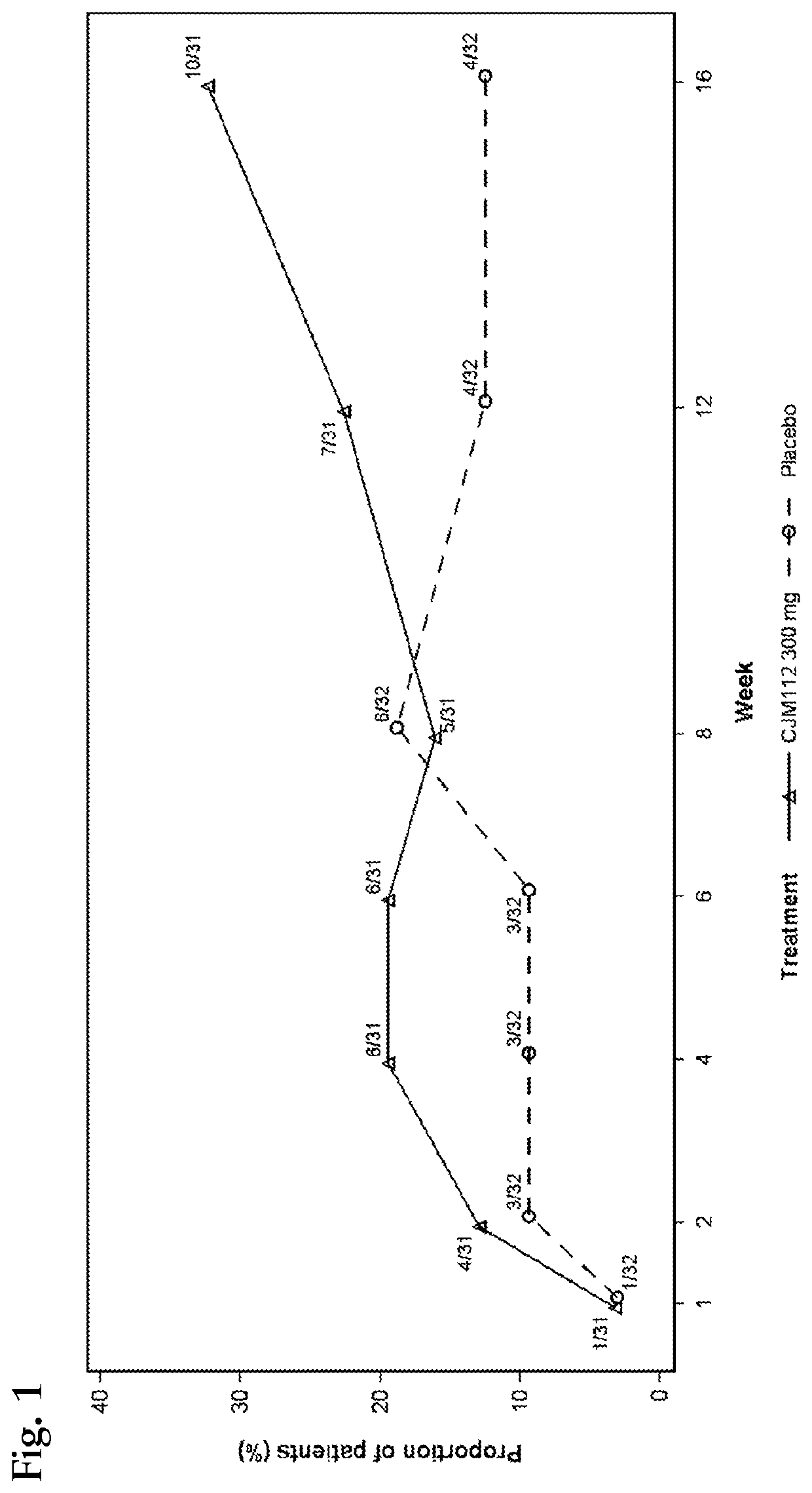
[0176]Early clinical evidence of the effects of an anti-IL-17 antibody, CJM112, supports the potential of an anti-IL-17 antibody as an effective therapy for patients with HS. Like secukinumab, CJM112 is a recombinant fully human anti-interleukin-17A monoclonal antibody of the IgG1 / κ isotype, developed for the potential treatment of autoimmune and inflammatory conditions. CJM112 binds with higher affinity to human homodimer IL-17A (6 pM) than secukinumab, and neutralizes the bioactivity of IL-17A in vitro.
[0177]This phase 2 study (CCJM112X2202) was a randomized, placebo controlled, double blind, multicenter study with two periods in patients with moderate to severe chronic HS in parallel groups conducted in USA, Denmark, Switzerland, Germany and the Netherlands. This study consisted of approximately 4 weeks screening period, two sequential treatment periods of 16 weeks (Period 1 and Extension Period 2) and approximately 12 weeks of follow-u...
example 2
Example 2A: Responder Rate Predictions in Heavy Subjects for Higher Dosage (450 mg) and More Frequent Dosing (Q2w) of Secukinumab
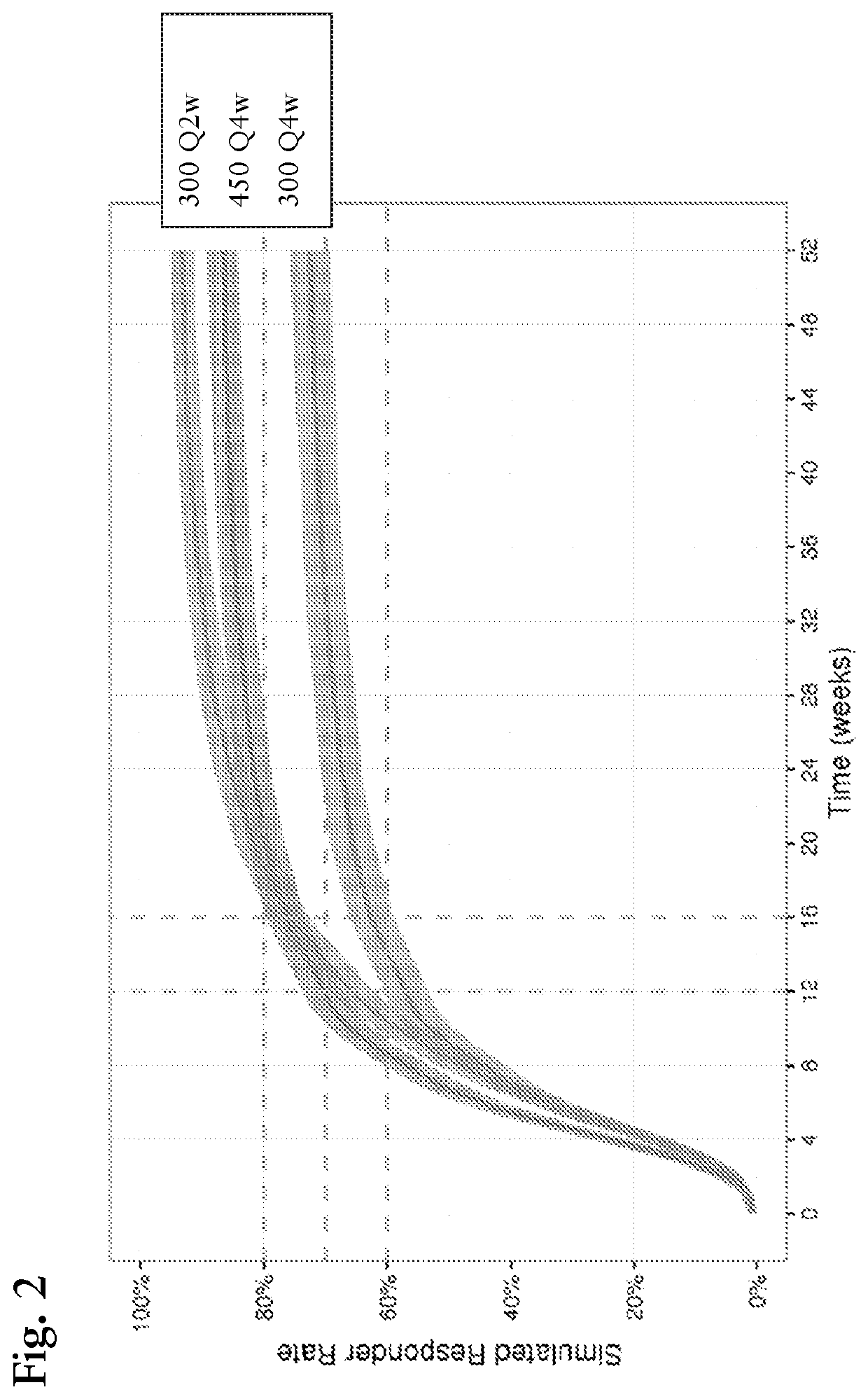
[0198]The purpose of the modeling and simulation (M&S) work in this Example is to investigate the simulated efficacy of secukinumab in heavy subjects following the two higher dosage regimens mentioned above, 450 Q4W and 300 Q2W. We report here modeling and simulation (M&S) work that investigated responder rate of PASI 75 and PASI 90 in patients ≥90 kg in bodyweight, using the standard regimen for secukinumab in psoriasis, i.e. 300 mg Q4W, in comparison to predicted response using higher dose regimens, i.e. 450 mg Q4W or 300 mg Q2W. The main objective of the work is to use model predicted (i.e. simulated) response rates to estimate the magnitude of improvement with the higher doses in heavier patients.
[0199]PASI data from studies CAIN457A2302 and CAIN457A2303 up to week 52 were used in this analysis. Only subjects ≥90 kg were used in the model building (n=6...
example 2b
b Dose-Response Modelling and Simulation for Heavy Patients
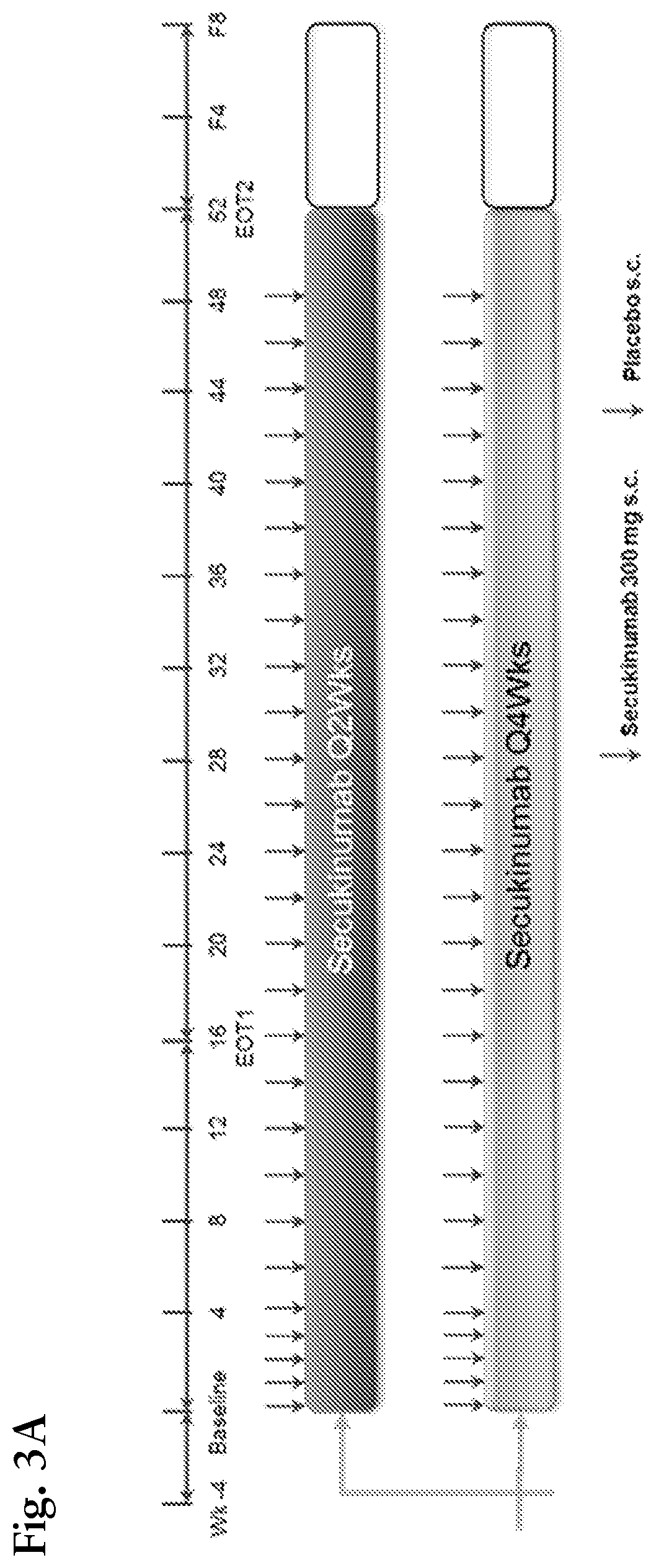
[0206]The modeling and simulation in this example consists of week 52 data from the secukinumab OPTIMIZE study. OPTIMIZE (NCT02409667) was a 52 week comparative, randomized, multicenter, open-label trial with blinded-assessment to evaluate the efficacy, safety and tolerability of secukinumab 300 mg SC in long-term treatment optimization in patients with moderate to severe chronic plaque-type psoriasis. In this study, suboptimal responders at Week 24, i.e., patients who reached PASI75 (i.e., a 75% reduction from baseline in PASI score) but did not reach PASI90 after 24 weeks under secukinumab 300 mg q4w were subsequently randomized to either secukinumab 300 mg q4w or secukinumab 300 mg q2w until Week 52.
[0207]The top panel of FIG. 5 displays the percentage of responders (Patients achieving PASI90, i.e., a 90% reduction from baseline in PASI score at Week 52) by treatment group (q2w or q4w) and weight category (90 kg) in that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com