DNA sequence that increases odorant receptor representation in the olfactory system
a technology of odorant receptors and dna sequences, which is applied in the field of genetically modified organisms with an increased representation of odorant receptors, can solve the problems of limited success in odor profiling or heterologous cell expression in vitro, many in vitro characterized or alleles may not be functional in an in vivo setting, and high cost of us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
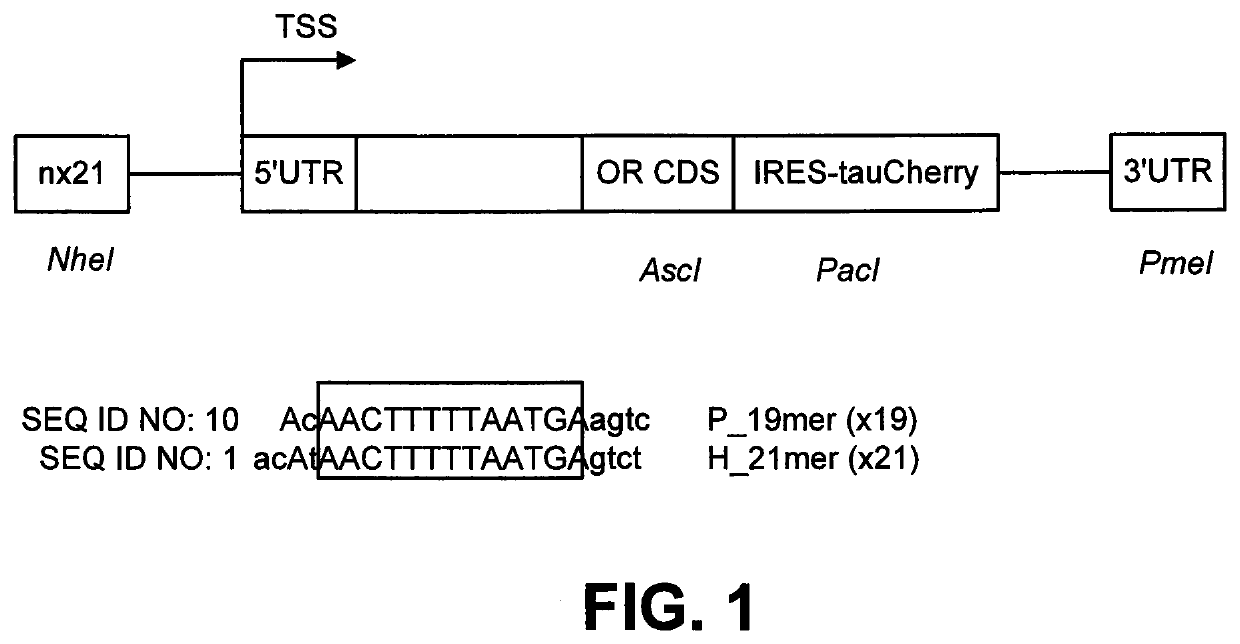
[0019]This disclosure pertains to a method of controlling the expression of specific odorant receptors in vivo in mice by multimerizing a specific 21 bp sequence encompassing the homeodomain binding site sequence, TAATGA, known to be a determinant in OR gene choice and adding it to the M71 odorant receptor (OR) transgene backbone (see Rothman et al. The Promoter of the mouse odorant receptor gene M71; Mol. Cell. Neurosci. 28, 535-546, 2005). Of note, the 21 bp sequence was chosen to reflect two complete turns of double stranded B-DNA (10.5 bp per turn on average). The method reproducibly and dramatically increases the total number of olfactory sensory neurons (OSNs) expressing specific mouse or human OR coding sequences in different transgenic animals. The transgenic vector was designed in such way that any odorant receptor or G-protein-coupled receptors (GPCR) (human, mouse or from any species), can be shuttled into the transgenic backbone. Importantly, proof of concept studies sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com