Treatment of hyperbilirubinemia
a technology of bilirubinemia and nucleic acid sequence, which is applied in the direction of transferases, peptide/protein ingredients, skeletal/connective tissue cells, etc., can solve the problems of loss of efficacy, potential exposure of patients to life-threatening spikes, and lethal disease, so as to improve the expression of transgenes and improve the immunogenicity of constructs. , the effect of reducing the risk of life-threatening spikes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0064]The invention is further described in detail by reference to the following experimental examples and the attached figures. These examples are provided for purposes of illustration only, and are not intended to be limiting.
Material and Methods
Codon Optimization and AAV Vector Construct:
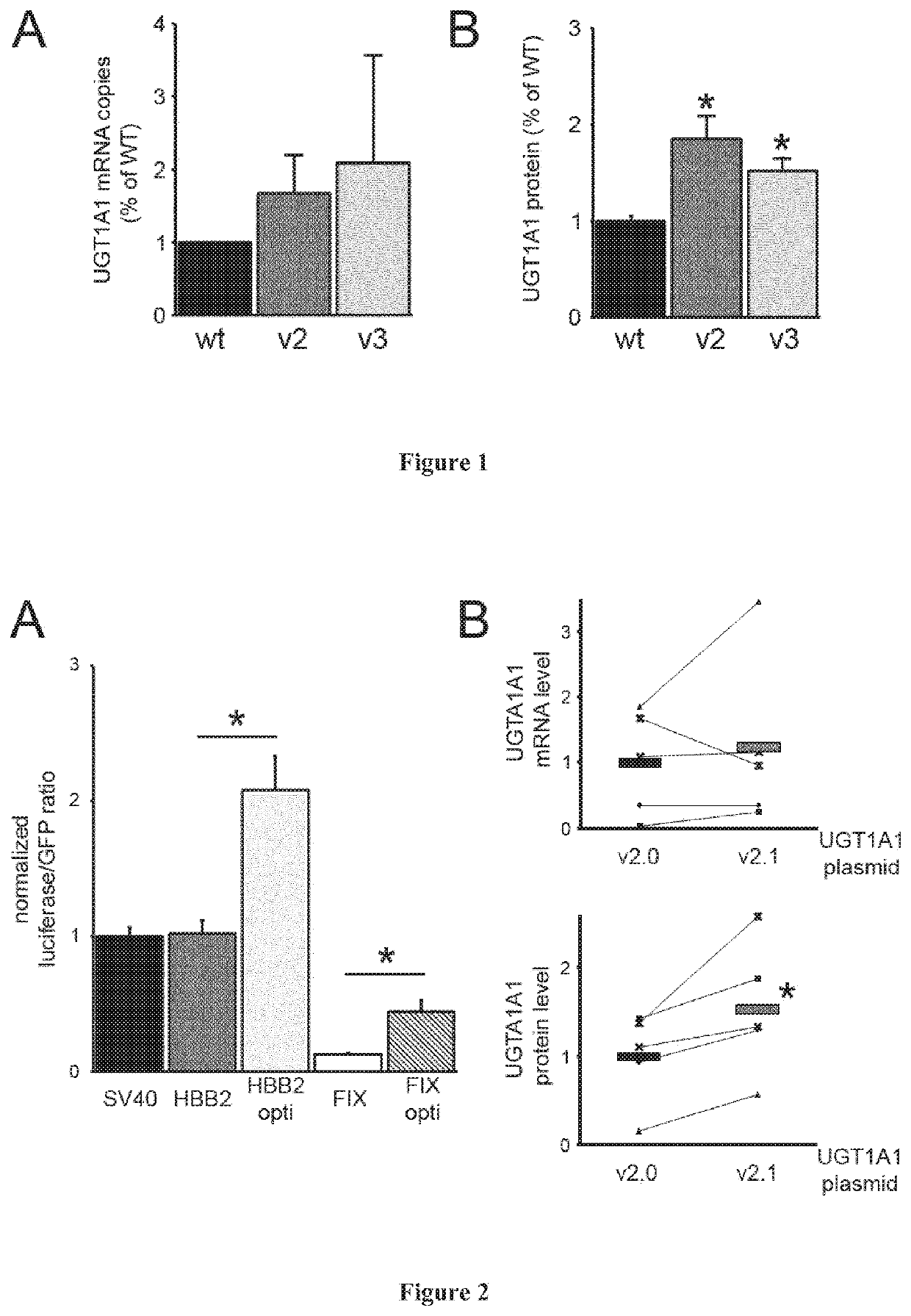
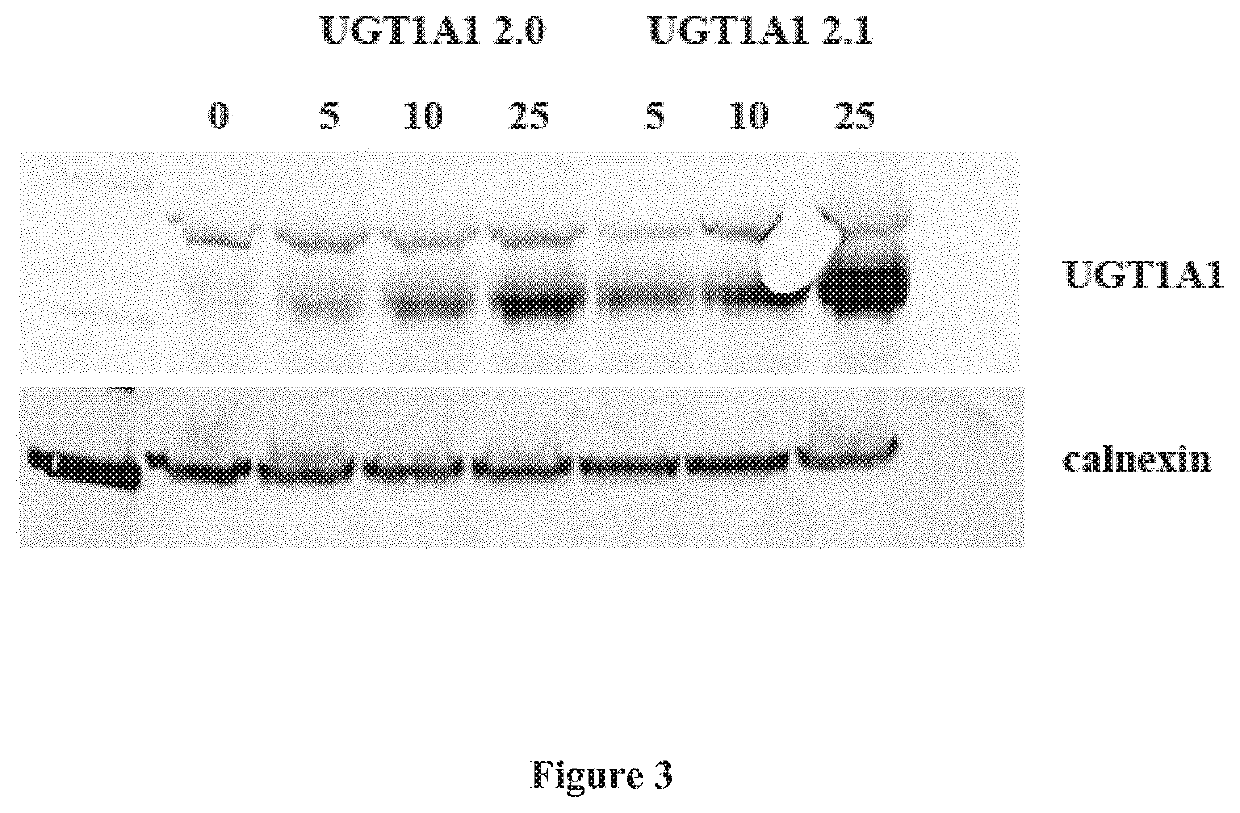
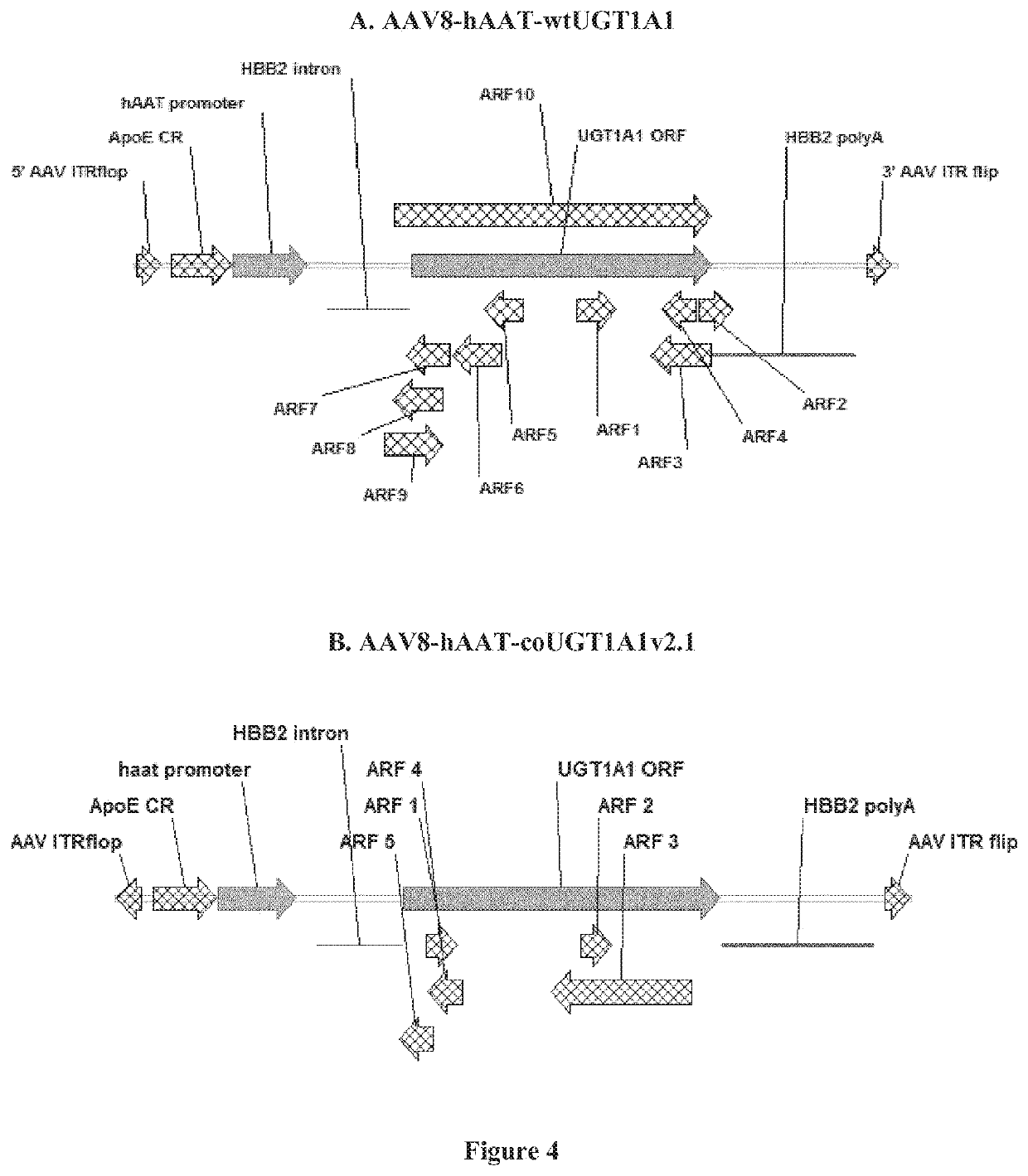
[0065]The UGT1A1 underwent codon optimization according to several different algorithms. Additionally, removal of cryptic transcription start sites was implemented throughout the construct. The resulting constructs were either introduced into expression plasmids, or packaged into AAV serotype 8 vectors and tested in vitro and in vivo (rats and mice) for potency.
[0066]The following abbreviations are used throughout this experimental part for these constructs:[0067]WT.0: wild-type UGT1A1 transgene and the wild-type HBB2 intron (SEQ ID NO:5);[0068]WT: wild-type UGT1A1 transgene and the optimized version of the HBB2 intron with some ARFs removed (SEQ ID NO:6);[0069]v2 (or v2.0): comprises codon-optim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com