Methods of Using Compositions Comprising Variants and Fusions of FGF19 Polypeptides for Treatment of Nonalcoholic Fatty Liver Disease
a technology of fatty liver disease and composition, which is applied in the direction of peptide/protein ingredients, metabolism disorders, instruments, etc., can solve the problems of reducing plasma total and ldl cholesterol, affecting the homeostasis of bile acids, and potentially toxic bile acids to cells. , to achieve the effect of improving bile acid homeostasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0259]The following is a description of various methods and materials used in the studies herein.
[0260]Animals.
[0261]db / db mice were purchased from The Jackson Laboratory (Bar Harbor, Me.), Mice were kept in accordance with welfare guidelines under controlled light (12 hr light and 12 hr dark cycle, dark 6:30 pm-6:30 am), temperature (22±4° C.) and humidity (50%±20%) conditions. Mice had free access to water (autoclaved distilled water) and were fed ad libitum on a commercial diet (Harlan Laboratories, Indianapolis, Ind., Irradiated 2018 Teklad Global 18% Protein Rodent Diet) containing 17 kcal % fat, 23 kcal % protein and 60 kcal % carbohydrate. All animal studies were approved by the NGM Institutional Animal Care and Use Committee.
[0262]DNA and Amino Acid Sequences.
[0263]cDNA of ORF encoding human FGF19 (Homo sapiens FGF19, GenBank Accession No. NM_005117.2) variants. Protein sequence encoded by the cDNA (GenBank Accession No. NP_005108.1).
[0264]PCR.
[0265]FGF19 ORF was amplified w...
example 2
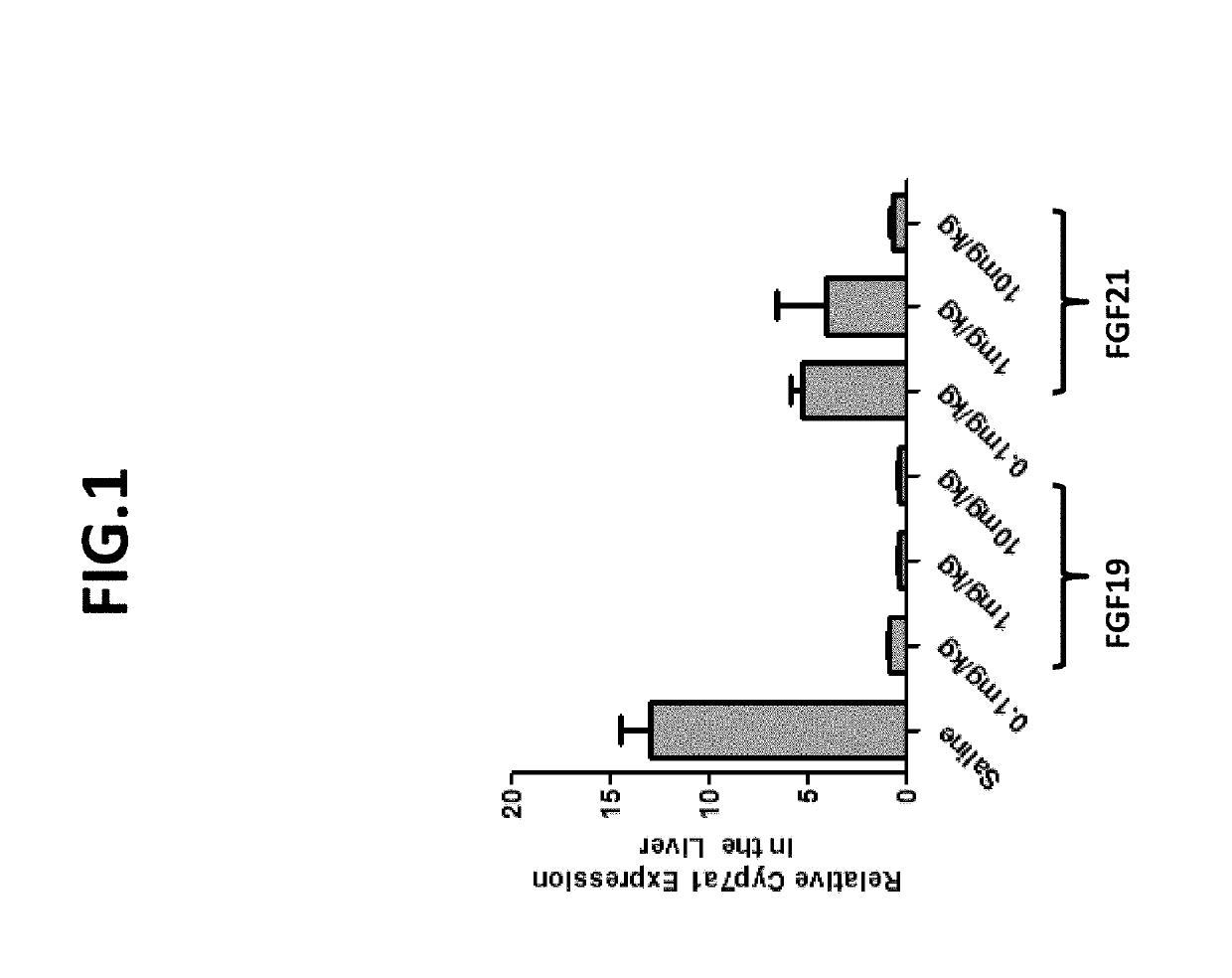
[0280]In order to confirm that FGF19 variants such as those set forth herein repress cyp7a1 expression, inhibition of cyp7a1 expression by wild-type FGF19 was determined following administration of various concentrations. The effects of FGF21 were assessed in a comparable manner.
[0281]Briefly, at time 0, db / db mice were dosed intraperitoneally with either recombinant FGF19 (0.1 mg / kg; 1 mg / kg; 10 mg / kg) or recombinant FGF21 (0.1 mg / kg; 1 mg / kg; 10 mg / kg). Five hours after dosing, livers were harvested, RNA was extracted, and cyp7a1 expression was determined by real-time PCR (QPCR) using GADPH as a normalization control. In each group of mice, n=3, and cyp7a1 expression values for the various FGF19 and FGF21 concentrations were compared to mice dosed with PBS vehicle control.
[0282]As set forth in FIG. 1, FGF19 dramatically decreased cyp7a1 expression in a concentration-dependent manner. Although administration of FGF21 caused a reduction of cyp7a1 expression, the effect was demonstra...
example 3
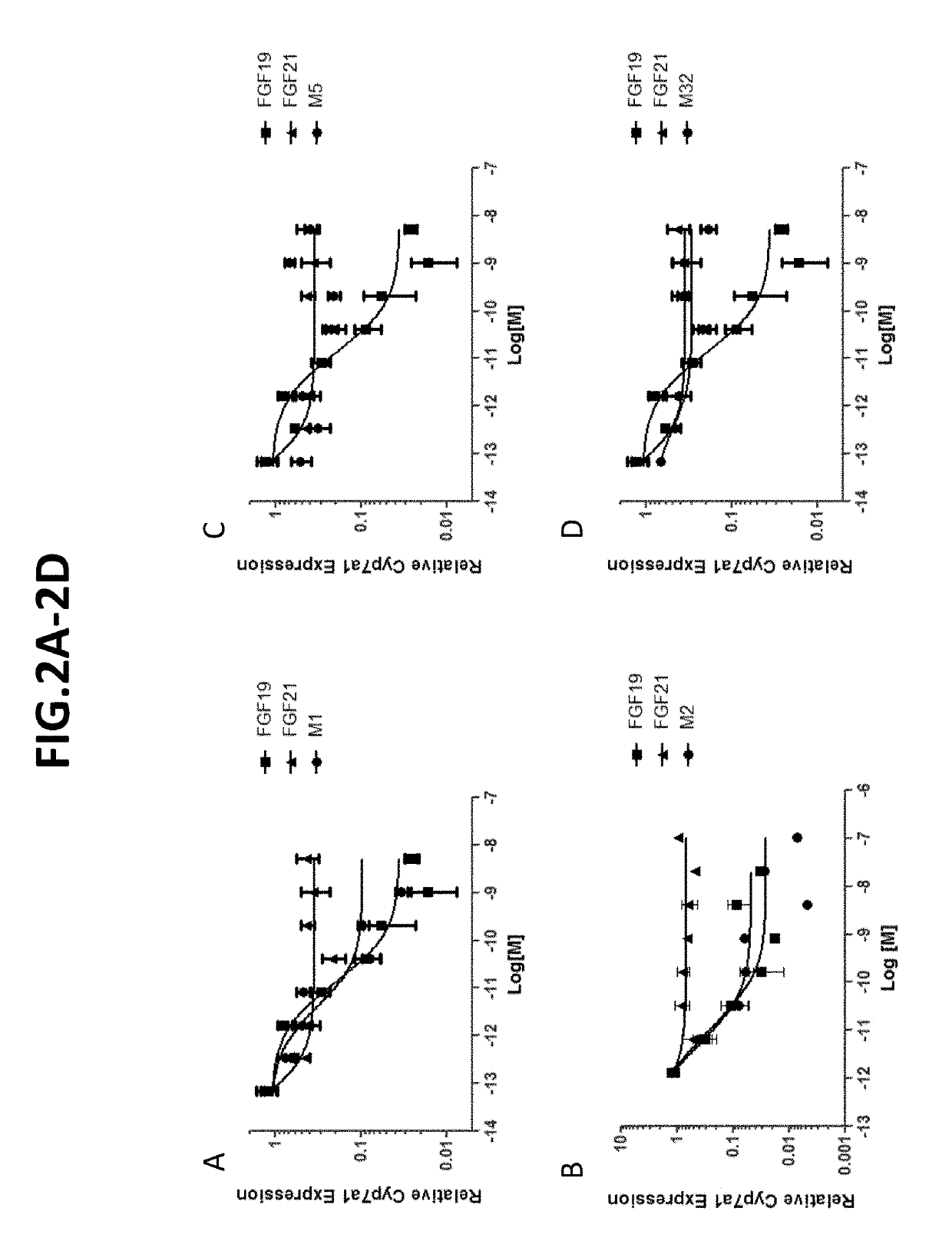
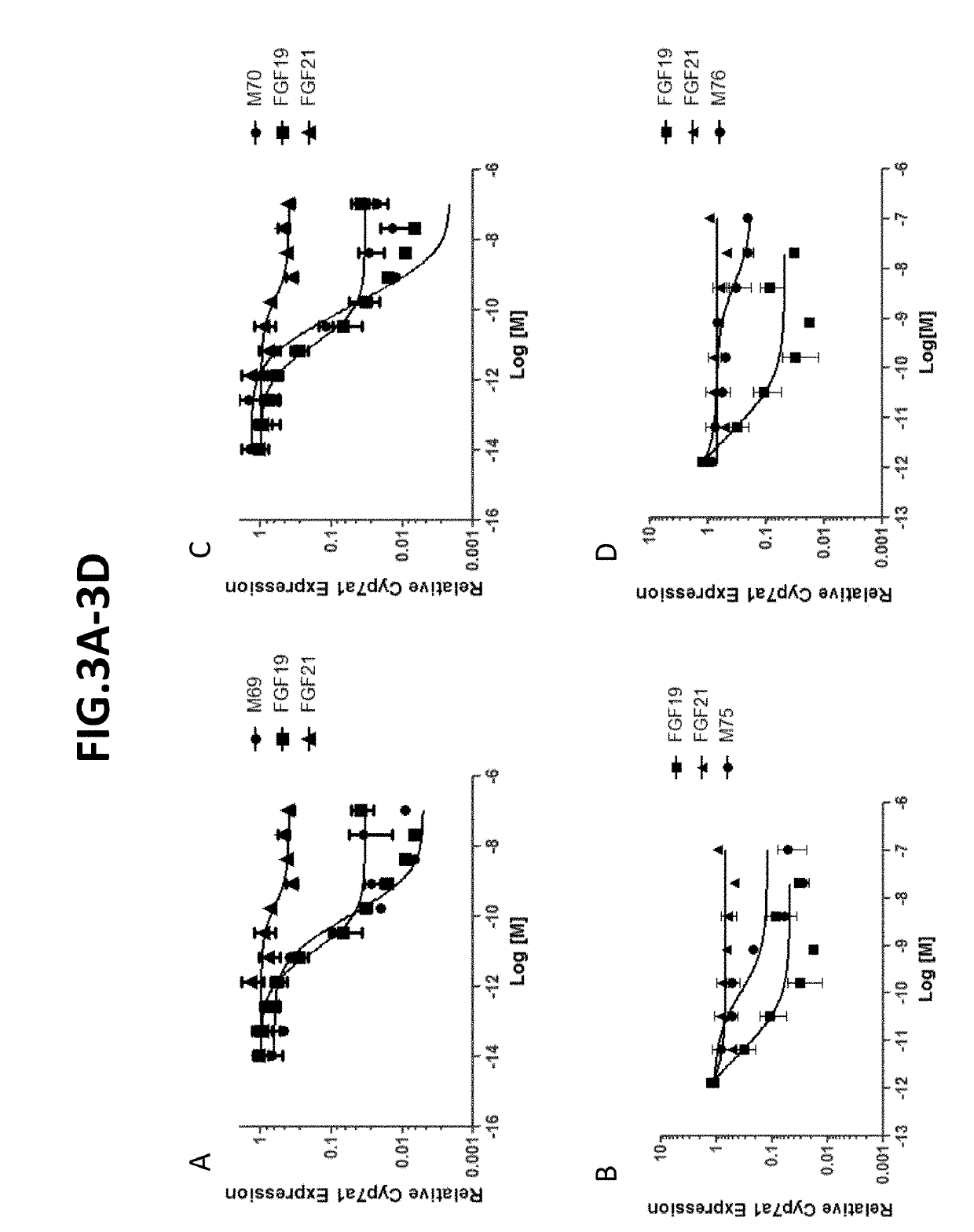
[0284]Using the assays described above, repression of cyp7a1 in primary human hepatocytes was determined for a number of FGF19 variants. As indicated in FIG. 3-FIG. 5, several variants (e.g., M1, M2, etc.) exhibited strong cyp7a1 repression.
[0285]To evaluate effects of some additional FGF19 variants on Cyp7a1 repression, the in vitro cell-based assay (primary human hepatocyte) and the in vivo assay (protein dosing in db / db mice) were utilized in which the variants were compared with saline-treated controls. FIG. 5 sets forth the results (IC50 and Cyp7a1(%)) in tabular form. While most FGF19 variants that were evaluated exhibited Cyp7a1-inhibiting activity, a few variants (e.g., M90, M96, M98, M5 and M32) no longer repressed Cyp7a1.
[0286]FGF19 variants that retain Cyp7a1 repression activity can be further evaluated in the HCC assay (or other relevant assay or model) described above to identify variants that might be useful for modulating bile acid metabolism and / or for treating bile ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure gradient | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com