Palatable compositions including sodium phenylbutyrate and uses thereof
a technology of sodium phenylbutyrate and composition, applied in the field of palatable compositions including sodium phenylbutyrate, can solve the problems of high bcaa level, accumulation of substrate, and various symptoms, and achieves the effects of chronic and acute neurological damage, high bcaa level, and high bcaa level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of Taste-Masked Formulations of Sodium Phenylbutyrate
[0162]Taste-masked materials may be prepared using the following methodology.
Drug Layering Solution
[0163]A solution of HPMC E 5 and PEG 6000 in purified water is made. A separate solution of sodium phenylbutyrate in purified water is also prepared. The two solutions are then combined to create the final drug layering solution of HPMC E 5, PEG 6000, and sodium phenylbutyrate in purified water.
[0165]Cellulose pellets are preheated to 35+ / −2° C. in a GPCG-1 fluid bed with a 6″ Wurster insert, and the drug layering solution is sprayed. Inlet air temperature is adjusted to maintain product temperature at 35-45 C during coating. After spraying, the coated pellets are dried for a minimum of 5 minutes at 40° C. The product is passed through 40#-70# screen, and stored in a polyethylene bag until the next solution is prepared.
Seal Coat Solution
[0166]A seal coat solution is prepared by mixing Opadry Clear in purifi...
example 2
on of a Taste-Masked Formulation of Sodium Phenylbutyrate
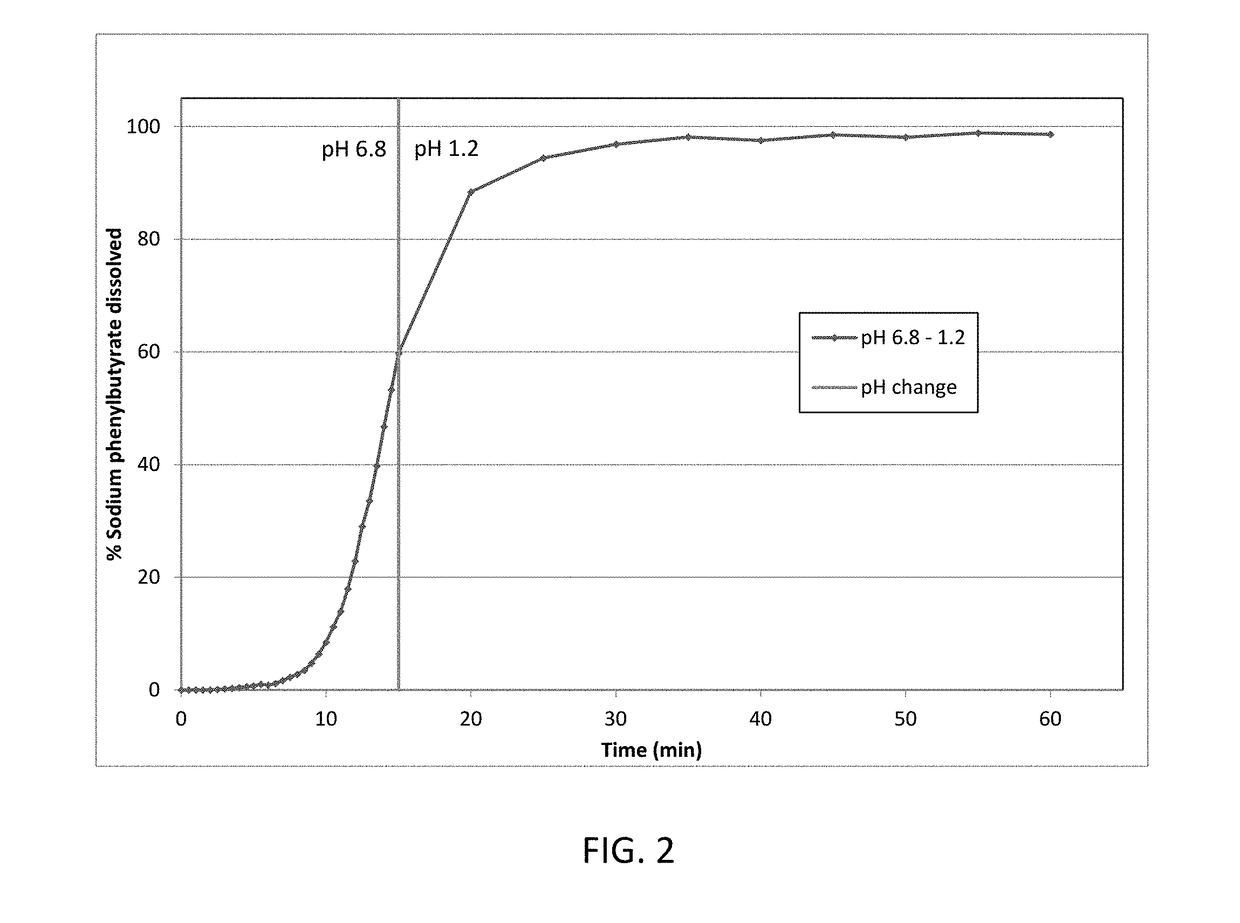
[0171]Taste-masked materials were prepared using the methodology described in Example 1 to achieve a formulation with a 44 wt % taste-mask coat and 16 wt % drug load. This formulation was dissolution tested as described in Example 1. The details of the formulation are presented in Table 3, and dissolution data is presented in FIG. 3.
TABLE 3Taste-mask coated formulation (44 wt% taste-mask coat, 16 wt % drug load)LayerIngredientmg / gSeed coreMicrocrystalline cellulose pellets325.3Drug layerSodium phenylbutyrate162.7HPMC E 540.7PEG 60004.1(H2O)—Seal coatOpadry Clear22.8(H2O)—Taste-mask coatEudragit E PO277.8PEG 600027.7Talc138.9(acetone)—(isopropyl alcohol)—
example 3
on of a Taste-Masked Formulation of Sodium Phenylbutyrate
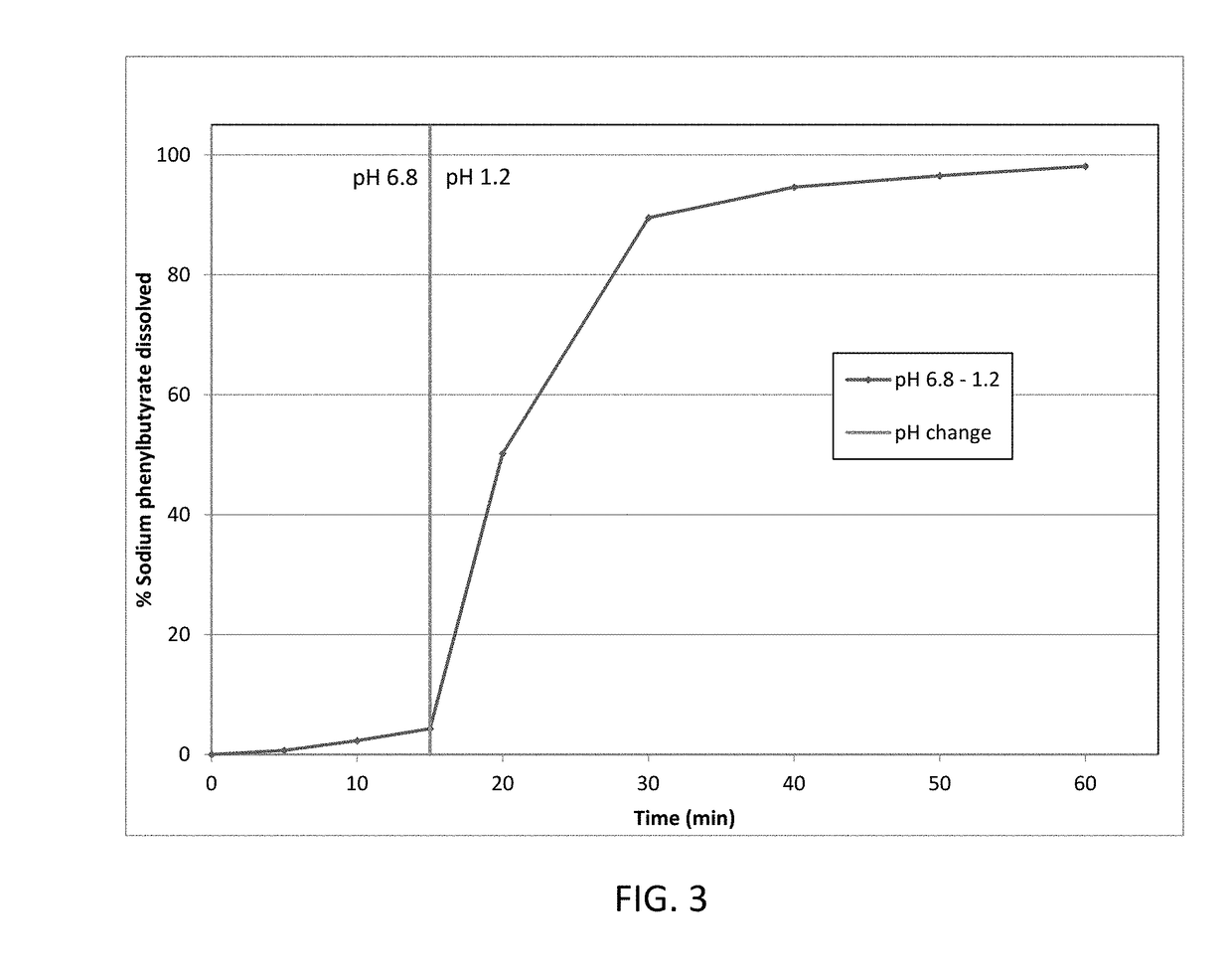
[0172]Taste-masked materials were prepared using the methodology described in Example 1, without the seal coat solution or coating steps, to achieve a formulation with a 31 wt % taste-mask coat and 47 wt % drug load. This formulation was dissolution tested as described in Example 1. The details of the formulation are presented in Table 4, and dissolution data is presented in FIG. 4.
TABLE 4Taste-mask coated formulation (31 wt% taste-mask coat, 47 wt % drug load)LayerIngredientmg / gSeed coreMicrocrystalline cellulose pellets161.4Drug layerSodium phenylbutyrate473.8HPMC E 547.4PEG 60007.1(H2O)—Taste-mask coatEudragit E PO206.9PEG 600020.6Talc82.8(acetone)—(isopropyl alcohol)—
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com