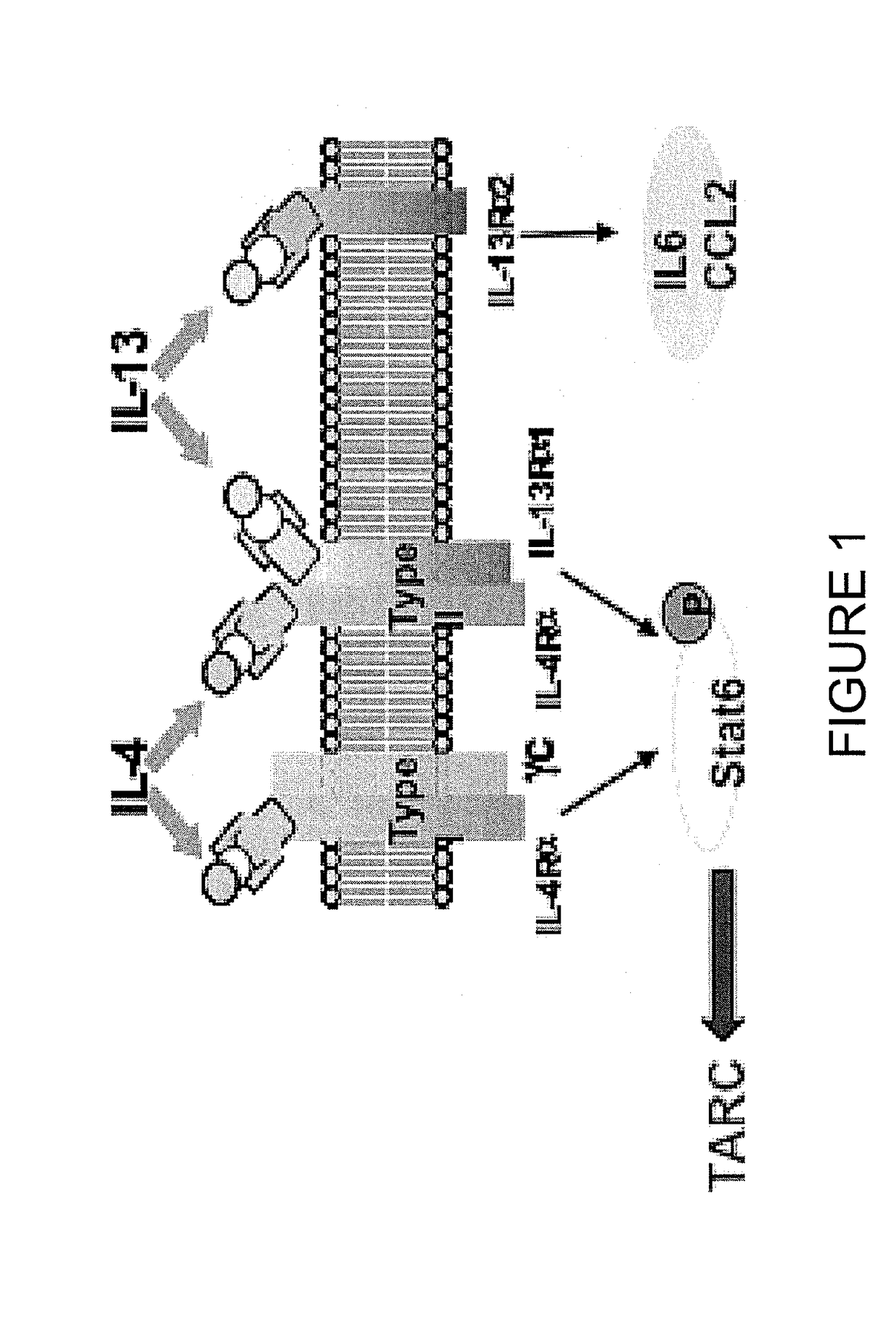
Anti-il4-il 13 bispecific antibodies
a technology of bispecific antibodies and anti-il4-il 13, which is applied in the field of anti-il4-il 13 bispecific antibodies, can solve the problems of delaying the progression of fibrosis in ipf patients, and achieve the effect of preventing the progression of fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Study Format
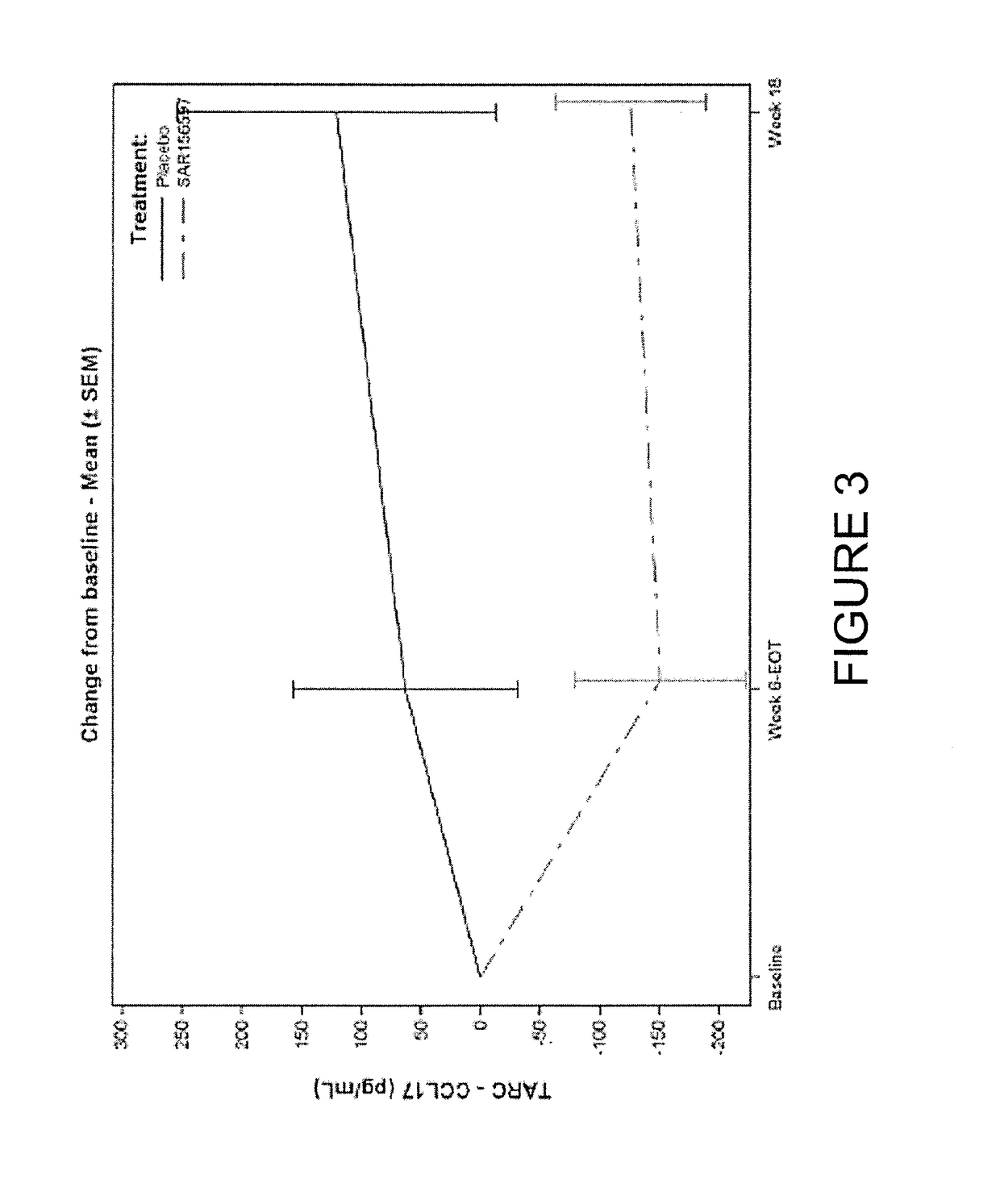
[0101]A multi-center, randomized, double-blind, placebo-controlled clinical study was conducted to assess the safety and tolerability of repeated doses of SAR156597 administered subcutaneously (SC) once weekly over a 6-week period in up to 3 sequential, ascending dose cohorts of patients with idiopathic pulmonary fibrosis (IPF). IPF is a progressive, diffuse, and distinct chronic fibrosing interstitial pneumonia of unknown cause that is uniformly fatal with a median survival of 2 to 3 years.
[0102]In each dose cohort, 8 patients (6 receiving SAR156597 and 2 receiving placebo) received repeated SC, once-weekly doses of SAR156597 or matching placebo control. The second cohort was initiated after the review of the safety of the first cohort by the DMC. The third cohort at a dose of 200 mg was initiated after the review of the safety of the 2 preceding cohorts.
[0103]For each patient, the study duration was 22 weeks, as follows: 4 weeks of screening; 6 weeks of treatment perio...
example 2
ynamics (PD) Evaluations
[0136]The effect of repeated ascending doses of SAR156597 was evaluated on pulmonary function tests (PFTs), respiratory symptoms, and on selected biomarkers. More specifically, the following PD variables were evaluated:
[0137]1. Pulmonary function test (PFT): Carbon monoxide diffusing capacity, corrected for hemoglobin (DLCO); Forced (expiratory) vital capacity (FVC); Forced expiratory volume over 1 second (FEV1); Total lung capacity (body plethysmography) (TLC); Residual volume (body plethysmography) (RV). Two PFTs were performed within 3 weeks prior to dosing. The first PFT was performed to screen the patient and the data from the second PFT (at the randomization visit) was averaged with the first one to establish a baseline value; these tests were performed on separate days. A PFT was also performed at Visit 8 (Week 6) / end-of-treatment (EOT) and Visit 14 (Week 18 / 12 weeks following the last dose of study treatment).
[0138]2. Oxygen saturation was assessed us...
example 3
of Safety Data
[0145]A safety evaluation was based upon the review of the individual values (clinically significant abnormalities), descriptive statistics (summary tables, graphics). All the safety analyses were performed using the safety population.
[0146]For all safety data, the observation period were divided into 3 phases:[0147]The pre-treatment phase, defined as the time between when the patients give informed consent and the first dose of IMP administration.[0148]The on-treatment phase, defined as the time from the first dose of IMP administration up to the Week 18 visit (included).[0149]The post-treatment phase, defined as the time after the Week 18 visit (excluded).
[0150]Adverse events were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) using version 16.1.
[0151]For clinical laboratory, vital signs, and ECG parameters, and the potentially clinically significant abnormalities (PCSAs) were analyzed using the PCSA list shown in Table 2.
TABLE 2Criteria...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com