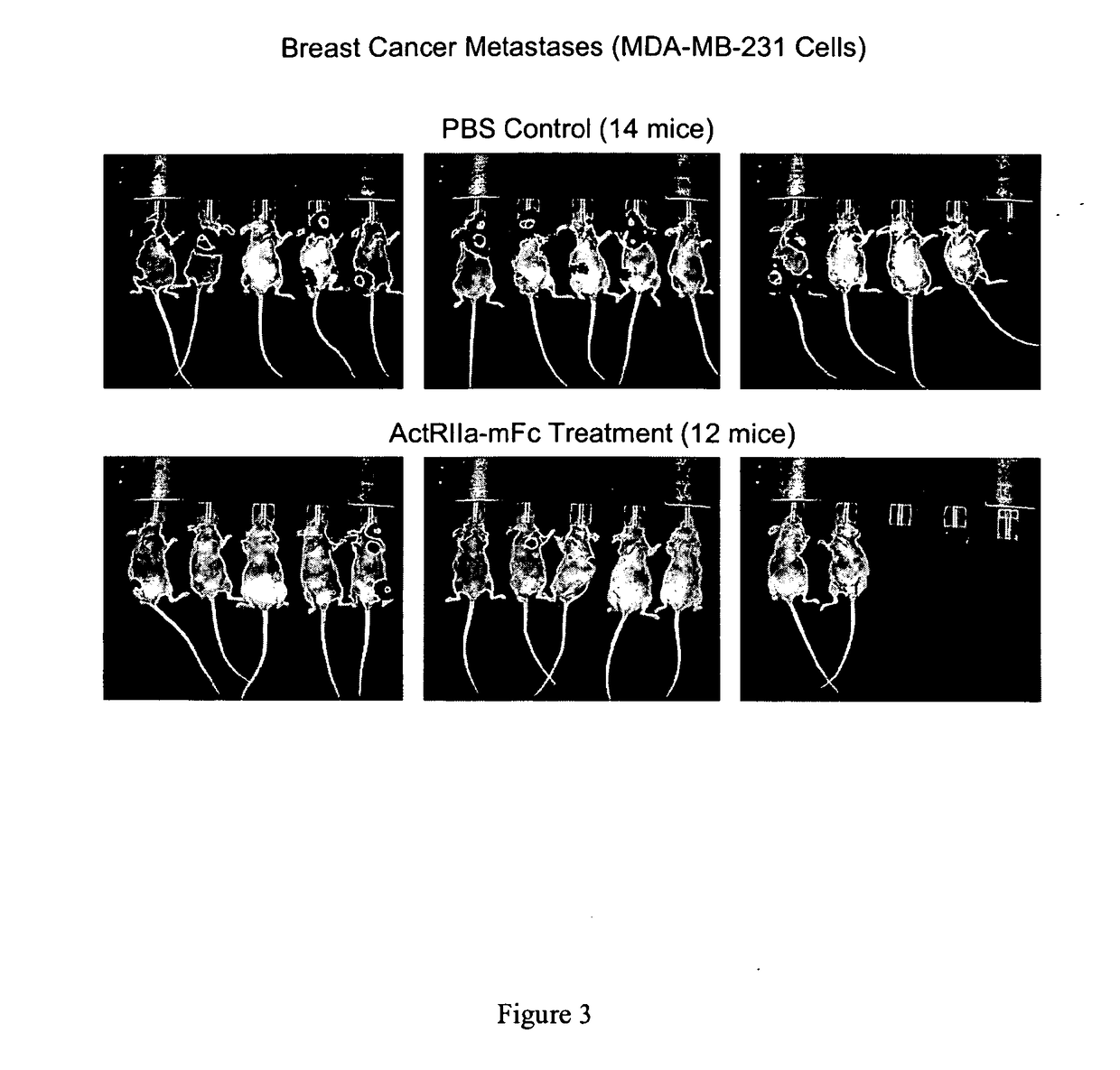
Activin-actriia antagonists and uses for treating or preventing breast cancer
a technology of activin-actriia and antagonists, which is applied in the direction of instruments, transferases, peptide/protein ingredients, etc., can solve the problems of increased fracture risk, increased treatment difficulty, and severe pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ActRIIa-Fc Fusion Proteins
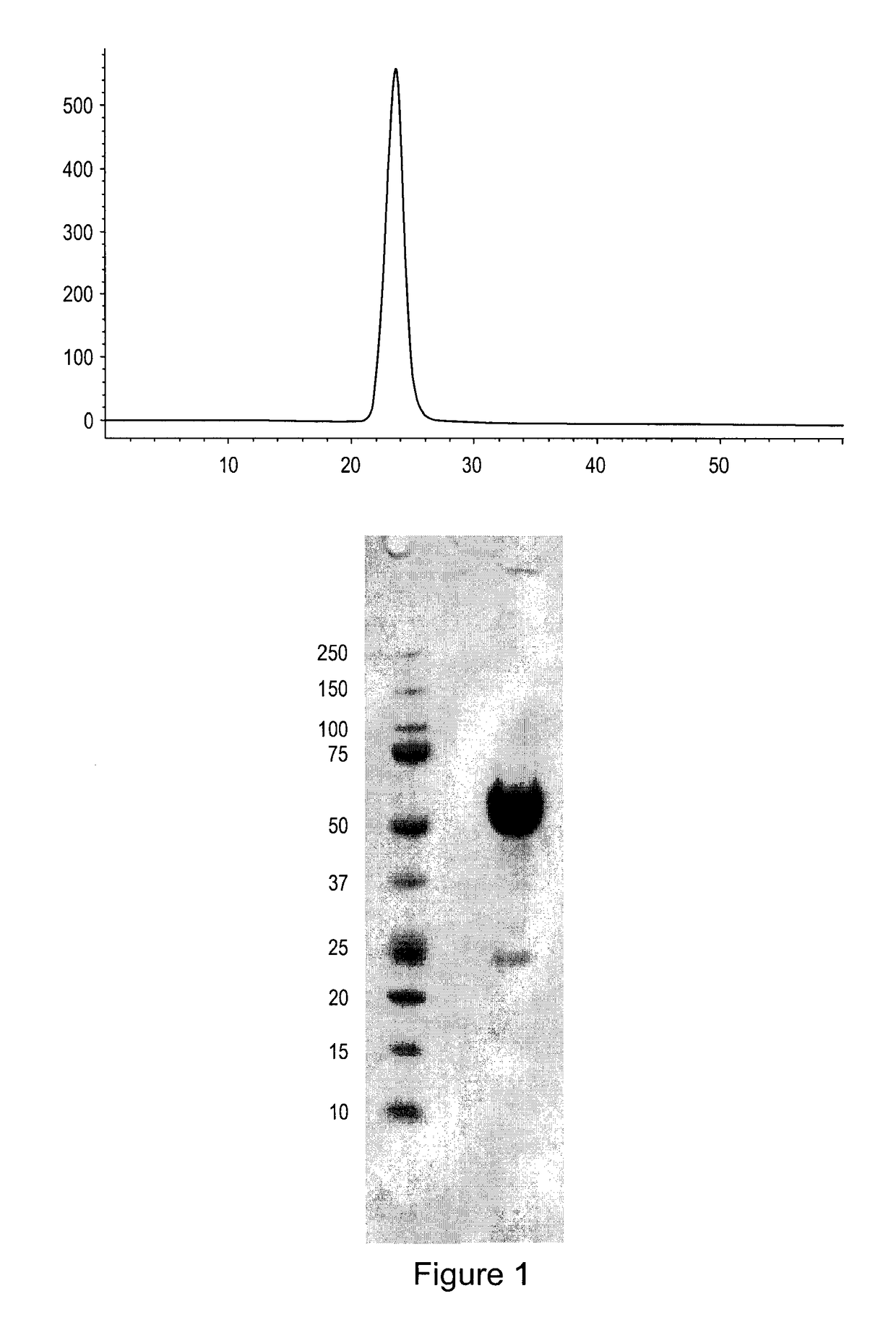
[0121]Applicants constructed a soluble ActRIIa fusion protein that has the extracellular domain of human ActRIIa fused to a human or mouse Fc domain with a minimal linker in between. The constructs are referred to as ActRIIa-hFc and ActRIIa-mFc, respectively.
[0122]ActRIIa-hFc is shown below as purified from CHO cell lines (SEQ ID NO: 7):
ILGRSETQECLFFNANWEKDRTNQTGVEPCYGDKDKRRHCFATWKNISGSIEIVKQGCWLDDINCYDRTDCVEKKDSPEVYFCCCEGNMCNEKFSYFPEMEVTQPTSNPVTPKPPTGGGTHTCPPCPAPELLGGPSVFLFPPKPKDT
[0123]The ActRIIa-hFc and ActRIIa-mFc proteins were expressed in CHO cell lines. Three different leader sequences were considered:
(i) Honey bee melitin (HBmL): (SEQ ID NO: 8)MKFLVNVALVFMVVYISYIYA,(ii) Tissue Plasminogen Activator (TPA): (SEQ ID NO: 9)MDAMKRGLCCVLLLCGAVFVSP,and(iii) Native: (SEQ ID NO: 10)MGAAAKLAFAVFLISCSSGA.
[0124]The selected form employs the TPA leader and has the following unprocessed amino acid sequence:
(SEQ ID NO: 13)MDAMKRGLCCVLLLCGAVFVSPGAAILGRSETQECLFFNANW...
example 2
Characterization of an ActRIIa-hFc Protein
[0129]ActRIIa-hFc fusion protein was expressed in stably transfected CHO-DUKX B11 cells from a pAID4 vector (SV40 ori / enhancer, CMV promoter), using a tissue plasminogen leader sequence of SEQ ID NO:9. The protein, purified as described above in Example 1, had a sequence of SEQ ID NO:7. The Fc portion is a human IgG1 Fc sequence, as shown in SEQ ID NO:7. Sialic acid analysis showed that the protein contained, on average, between about 1.5 and 2.5 moles of sialic acid per molecule of ActRIIa-hFc fusion protein.
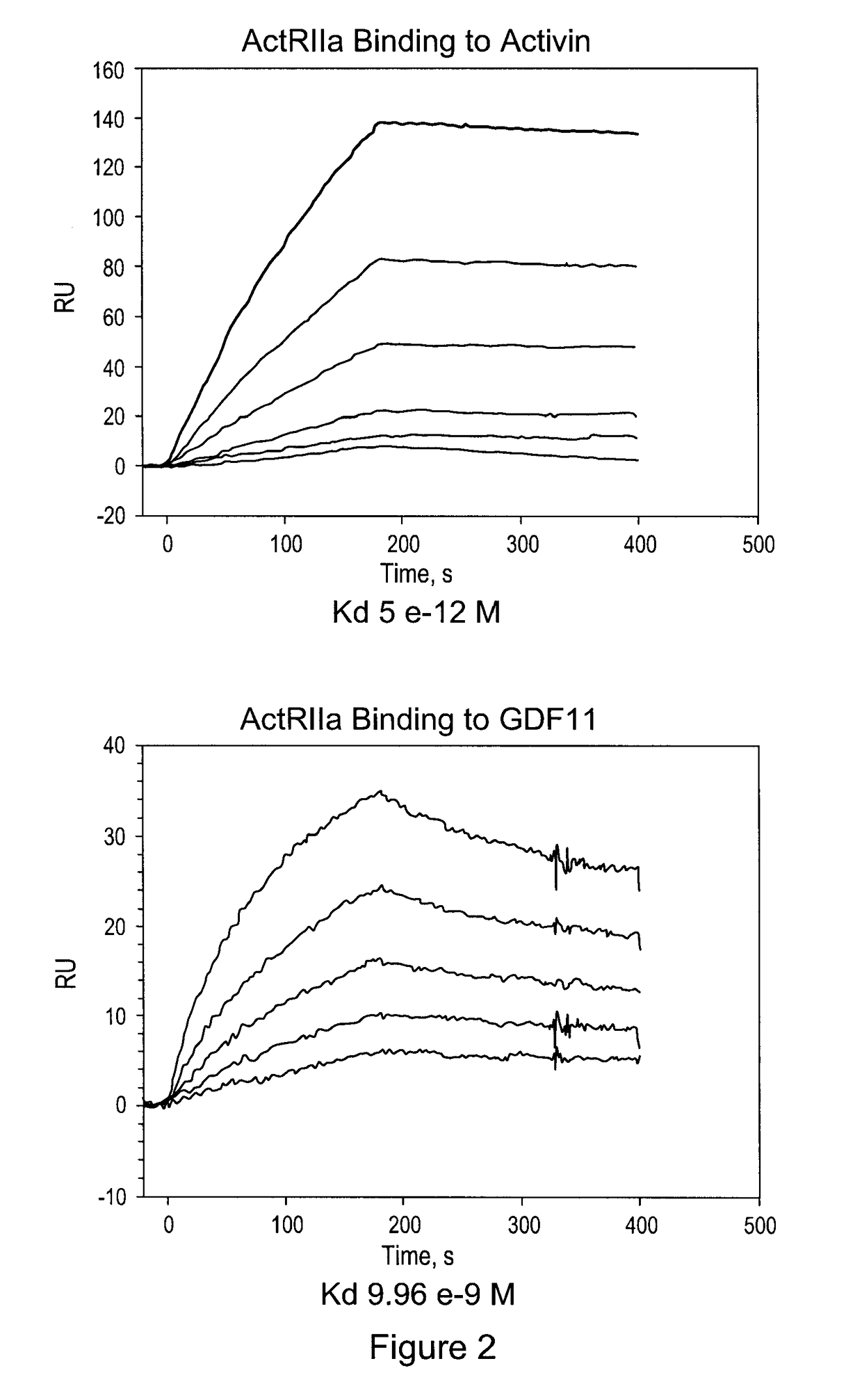
[0130]This purified protein showed a remarkably long serum half-life in all animals tested, including a half-life of 25-32 days in human patients (see Example 3, below). The CHO cell expressed material has a higher affinity for activin B ligand than that reported for an ActRIIa-hFc fusion protein expressed in human 293 cells (del Re et al., J Biol Chem. 2004 Dec. 17; 279(51):53126-35.). Additionally, the use of the tPa leader sequence p...
example 3
Human Clinical Trial
[0131]The protein described in Example 2 was administered to human patients in a randomized, double-blind, placebo-controlled study that was conducted to evaluate, primarily, the safety of the protein in healthy, postmenopausal women. Forty-eight subjects were randomized in cohorts of 6 to receive either a single dose of ActRIIa-hFc or placebo (5 active:1 placebo). Dose levels ranged from 0.01 to 3.0 mg / kg intravenously (IV) and 0.03 to 0.1 mg / kg subcutaneously (SC). All subjects were followed for 120 days. Subjects were excluded from study participation if they took medications affecting bone metabolism within 6 months of study entry. Safety evaluations were conducted following each cohort to determine dose escalation. In addition to pharmacokinetic (PK) analyses, the biologic activity of ActRIIa-hFc was also assessed by measurement of biochemical markers of bone formation and resorption, and FSH levels.
[0132]No serious adverse events were reported in this study...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com