Novel biological detection method for dioxins in serum, and a diagnostic use therefor in metabolic syndrome and related conditions
a biological detection and dioxin like technology, applied in the field of new biological detection methods for dioxin like activity in serum, can solve the problems of high price, compound is not easily decomposed or excreted in general, and accumulates in human body, so as to improve accuracy and ease, the effect of saving time and effor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ion of a Cell Line Expressing a Recombinant Reporter Gene Stably
Construction of a Recombinant Reporter Gene Vector
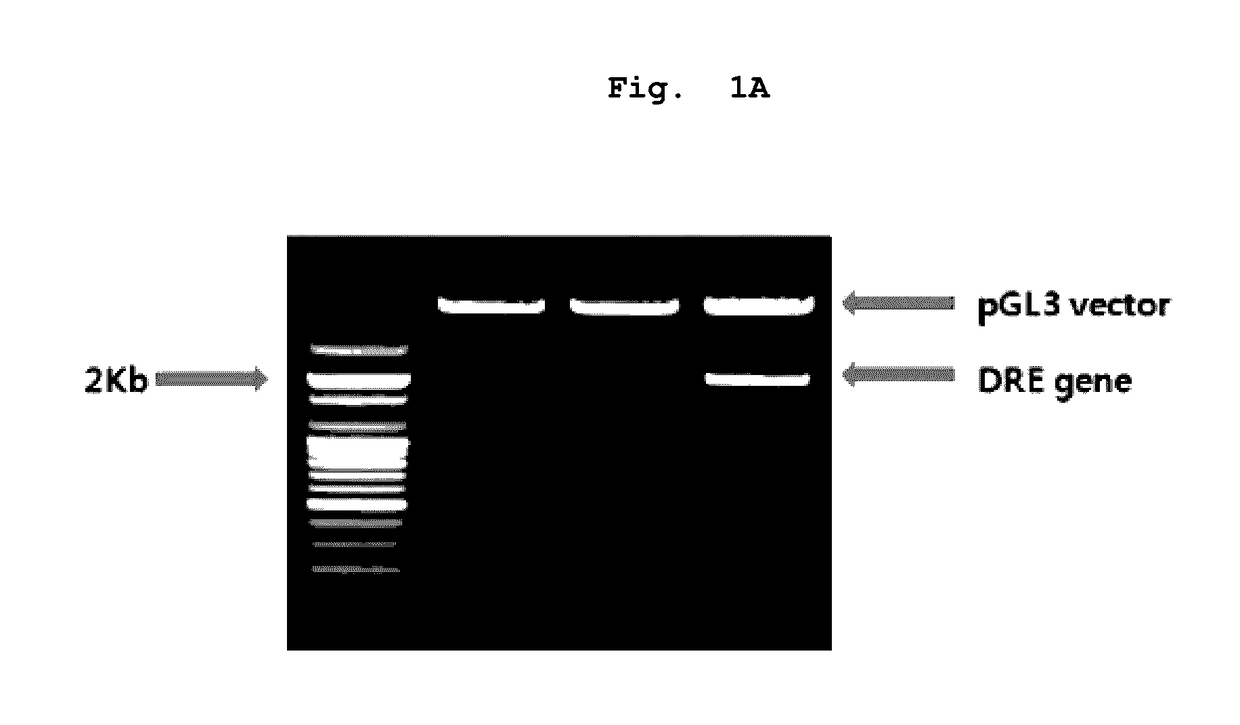
[0089]1.8 kb virus promoter fragment comprising mouse CYP1A1 promoter (482 bp) having 4 DRE binding sites (AhR binding sites) (5′-TNGCGTG-3′) and long terminal repeat (LTR) of mouse mammary tumor virus (MMTV) excluding glucocorticoid response element was cut out from pGudLuc1.1 vector by using Hind III, which was cloned in Hind III site of pGL3-basic vector digested with Hind III. As a result, pCYP1A1-luc vector was constructed (Han et al., BioFactors, 20:11-22, 2004). The direction of the promoter was confirmed by sequencing (FIG. 1B).
Construction of a Transformed Cell Line Expressing the Recombinant Reporter Gene
[0090]Hepa1c1c7, the mouse hepatocarcinoma cell line, was distributed in a 6 well plate (1×105 cells / well), followed by culture for 24 hours until the confluency reached 50%. 2 μg of pCYP1A1-luc vector constructed in Example and 0.5 μg of pcDNA3.1 vector wer...
example 2
Test with the Transformed Cell Line Expressing the Recombinant Reporter Gene
Response Test Using Indole-3-Carbinol
[0091]The cell line stably expressing pCYP1A1-luc constructed in Example was distributed in 60 mm culture dish at the density of 1×105 cells, followed by culture for 48 hours. Upon completion of the culture, the medium was replaced with DMEM supplemented with 0.5% charcoal-stipped FBS but not supplemented with phenol red, and indole-3-carbinol was treated thereto at the concentrations of 0, 0.01, 0.1, 1, 10, and 100 μM for 4, 8, or 24 hours. Then, the cells were collected, followed by luciferase assay.
[0092]As a result, as shown in FIG. 3, luciferase activity of the group treated with 0.001˜10 μM of indol-3-carbinol was increased dose-dependently, compared with that of the non-treated control group. In particular, when indol-3-carbionol was treated at the concentration of 100 μM, luciferase activity was increased at least 3 times. At this time, significant difference ov...
example 3
of Dioxins in Human Serum
Preparation of Human Serum Samples
[0096]Serum samples obtained from 97 people were heat-inactivated at 65° C. for 30 minutes. This heat-inactivation process secures the safety of cell culture.
Detection of Dioxins in Human Serum and Analysis of the Correlation Between Dioxins and Physical Variables
[0097]The cell line stably expressing pCYP1A1-luc constructed in Example was distributed in a 96 well plate at the density of 5×104 cells / well, followed by culture for 24 hours. The medium was replaced with DMEM not containing phenol red (90 μl / well), to which 10% human serum sample was treated (10 μl / well) for 24 hours. Then, the cells of each well were lysed by using luciferase lysis buffer (Promega). The lysate of each well was transferred into a 96 well plate (1 μl / well), followed by protein quantification by BCA method. The remaining cell lysates proceeded to luciferase assay using Promega luciferase assay kit. The luciferase activity was modified with prote...
PUM
| Property | Measurement | Unit |
|---|---|---|
| resistance | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
| mitochondria membrane potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com