Helical polycarbodiimide polymers and associated imaging, diagnostic, and therapeutic methods
a polymer and polymer technology, applied in the field of compositions comprising polycarbodiimide polymers, can solve the problems of limiting the ability to measure (e.g., via imaging) the kinetics of dynamic self-assembly and disassembly of photoluminescent nanotubes into live cell nuclei, and achieves the effects of convenient radiopharmaceutical delivery, convenient modification, and precise control of sub-cellular localization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
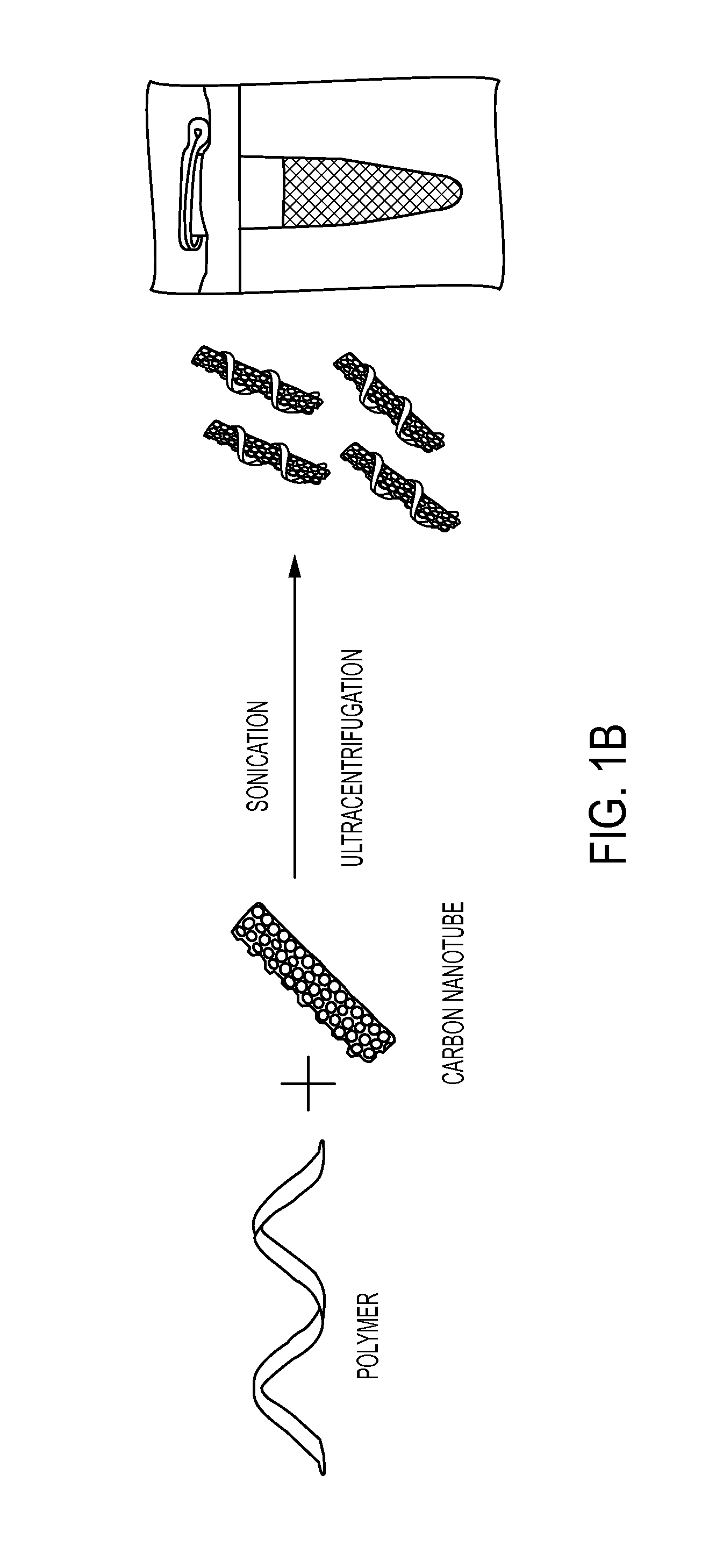
Helical Polycarbodiimide Cloaking of Carbon Nanotubes
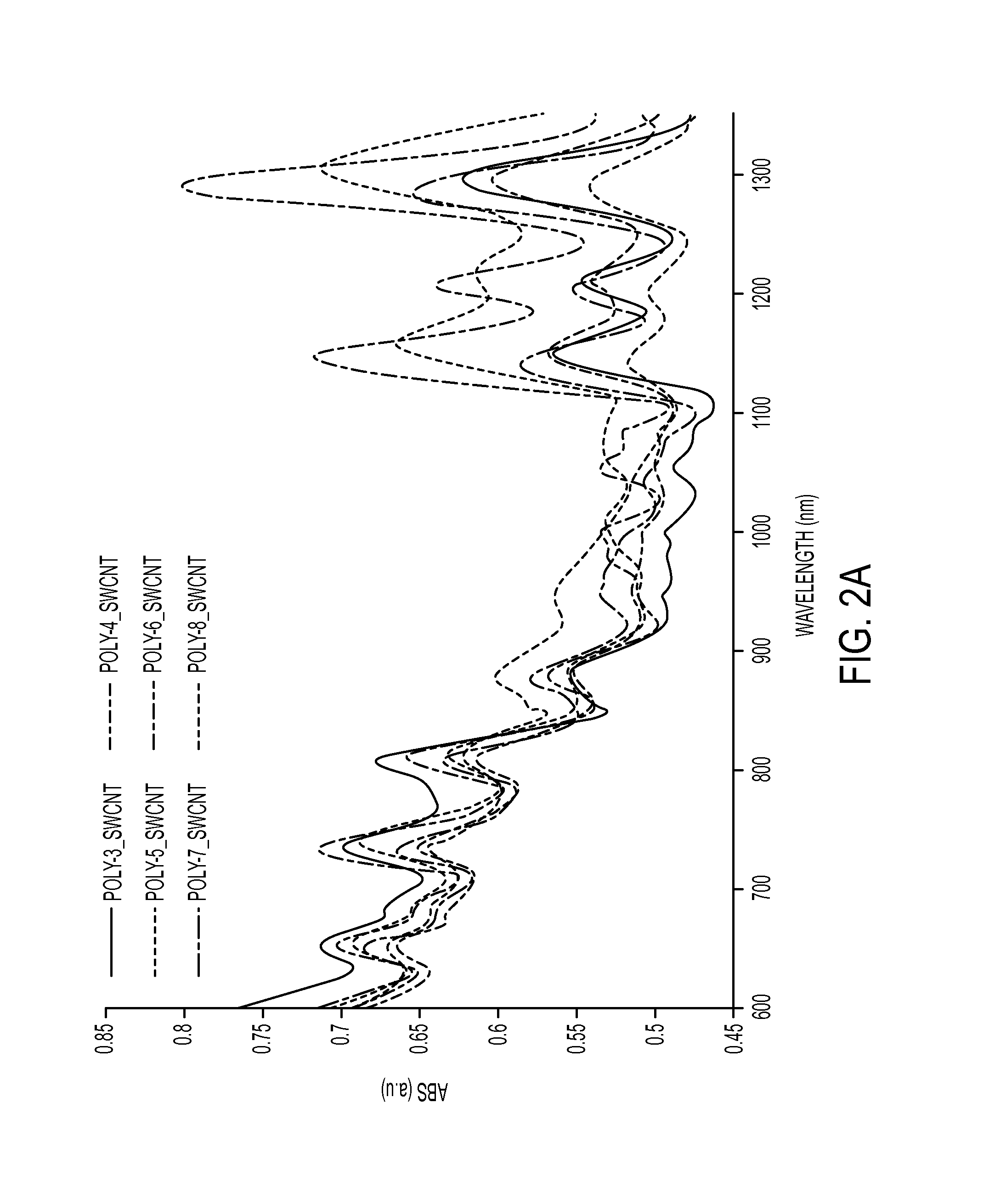
[0136]In the examples described herein, a platform of helical polycarbodiimide polymers was synthesized to ‘cloak’ the nanotubes which affected control over nanotube emission, provided a new mechanism of environmental responsivity, and enabled precise control over sub-cellular localization. The helical polymers exhibited ordered surface coverage on the nanotubes, allowed systematic modulation of nanotube optical properties, and produced up to 12-fold differences in photoluminescence efficiency. The polymers facilitated controllable and reversible inter-nanotube Förster resonance energy transfer, allowing kinetic measurements of dynamic self-assembly and disassembly. Tailored polycarbodiimide substituent groups also enabled sub-cellular targeting for imaging, including stable translocation of photoluminescent nanotubes within live cell nuclei.
[0137]Synthetic helical polymers mimic the basic structural motifs of vital biomolecules s...
example 2
Synthesis of Helical Polycarbodiimide Polymers, for Use in Therapeutic and Diagnostic Applications
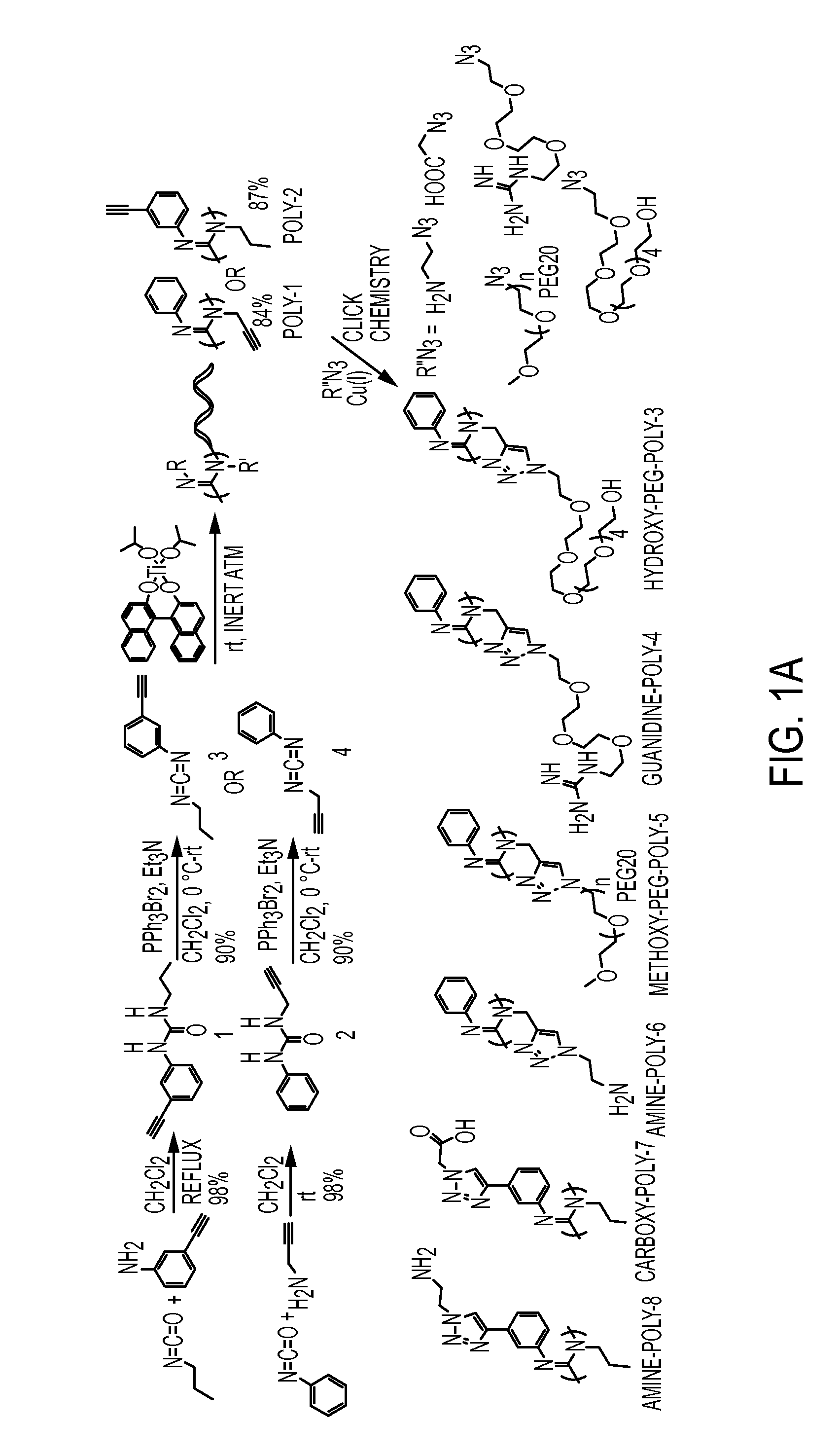
[0149]A synthesis scheme and molecular structures for helical polycarbodiimide polymers described herein are presented in FIGS. 12A-12C.
[0150]For synthesis of urea derivatives, a primary amine compound (RNH2) (1.0 equiv) was diluted in anhydrous dichloromethane and added to an isocyanate compound (RNCO) (1.2 equiv) in dichloromethane, stirred at low temperature, and kept cold in an ice bath. The reaction mixture was stirred at room temperature or refluxed overnight until the completion of the reaction. The solvent was removed in a rotary evaporator and crude white solid was purified by recrystallization in ethanol at 4° C. and dried to obtain white crystalline solid.
[0151]For synthesis of carbodiimide monomers, triethyl amine (2.5 equiv) was added to a suspension of dibromotriphenylphosphorane (1.2 mol equiv) in dichloromethane at low temperature and the reaction mixture was stirred at ...
example 3
Nanoscale Sensors for Quantitative Redox Potential Measurement
[0155]Reduction potential (or Redox) is a physical concept used to measure the tendency of chemical compounds (couples) to transfer electrons during a reaction, and by extension the chemical potential energy in a system or couple. The direction, regulation, and capacity for cellular activity depends upon the state of these redox reactions, quantifiable with an electric potential voltage, for phenomena as diverse as energy production, biosynthesis, gene expression, signaling and detoxification. Redox Biology currently remains largely qualitative. Recent linkage between perturbations in redox state and cancerous transformation, cell growth and division, cell viability, drug efficacy, and numerous pathologies have increased interest in quantitative Redox Biology.
[0156]In certain embodiments, the compositions described herein allow for a Single-Walled Carbon Nanotube (SWCNT) based optical sensor for this purpose. Current art ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| center-to-center distance | aaaaa | aaaaa |
| reduction potential | aaaaa | aaaaa |
| height projection near | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com