Chromium complexes as enhancers of brain glucose transporters
a technology of brain glucose transporter and chromium complex, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of chromium picolinate producing modest weight loss and body composition changes, biologically ineffective insulin and glucose metabolism, and excessive amounts of acetyl-coa and ketone bodies, so as to improve brain glucose transporter and alter brain glucose transport function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Chromium Increases Brain Glucose Transporter Levels in Subjects with Obesity and / or Insulin Resistance
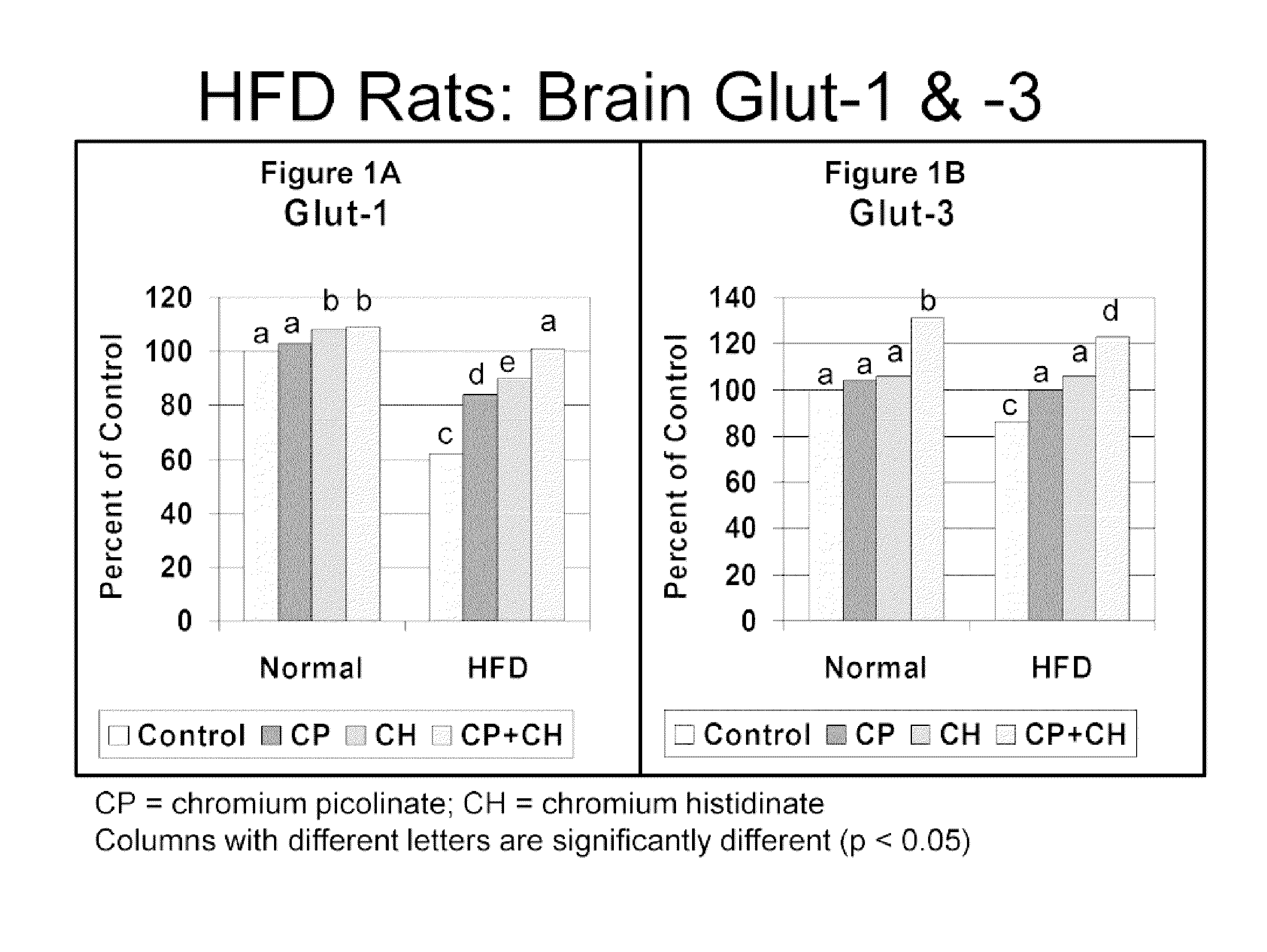
[0098]In order to test whether chromium affected insulin-resistance induced decreases in brain glucose transporter GLUT1 and GLUT3 levels, the levels of GLUT1 and GLUT3 expression products were compared in insulin-resistant or hyperglycemic animals, with and without administration of chromium.
[0099]Briefly, insulin resistance and hyperglycemia were induced in male Wistar rats by feeding a high fat diet (HFD, 40% of calories as fat). A healthy control group received a standard diet (12% of calories as fat). Rats were fed their diets throughout the 12 week study.
[0100]Rats (10 / group) were divided into groups: healthy control (Control), HFD, and HFD+CrHis. Chromium (Cr) treated rats were fed CrHis (110 mcg / kg body wt / d). Cr treatment started at the beginning of the study and was continued for 12 weeks. After 12 weeks, chromium levels and GLUT-1 and GLUT-3 expression (Western blot analy...
example 2
Chromium Increases Brain Glucose Transporter Levels in Subjects with Diabetes and / or Insulin Resistance
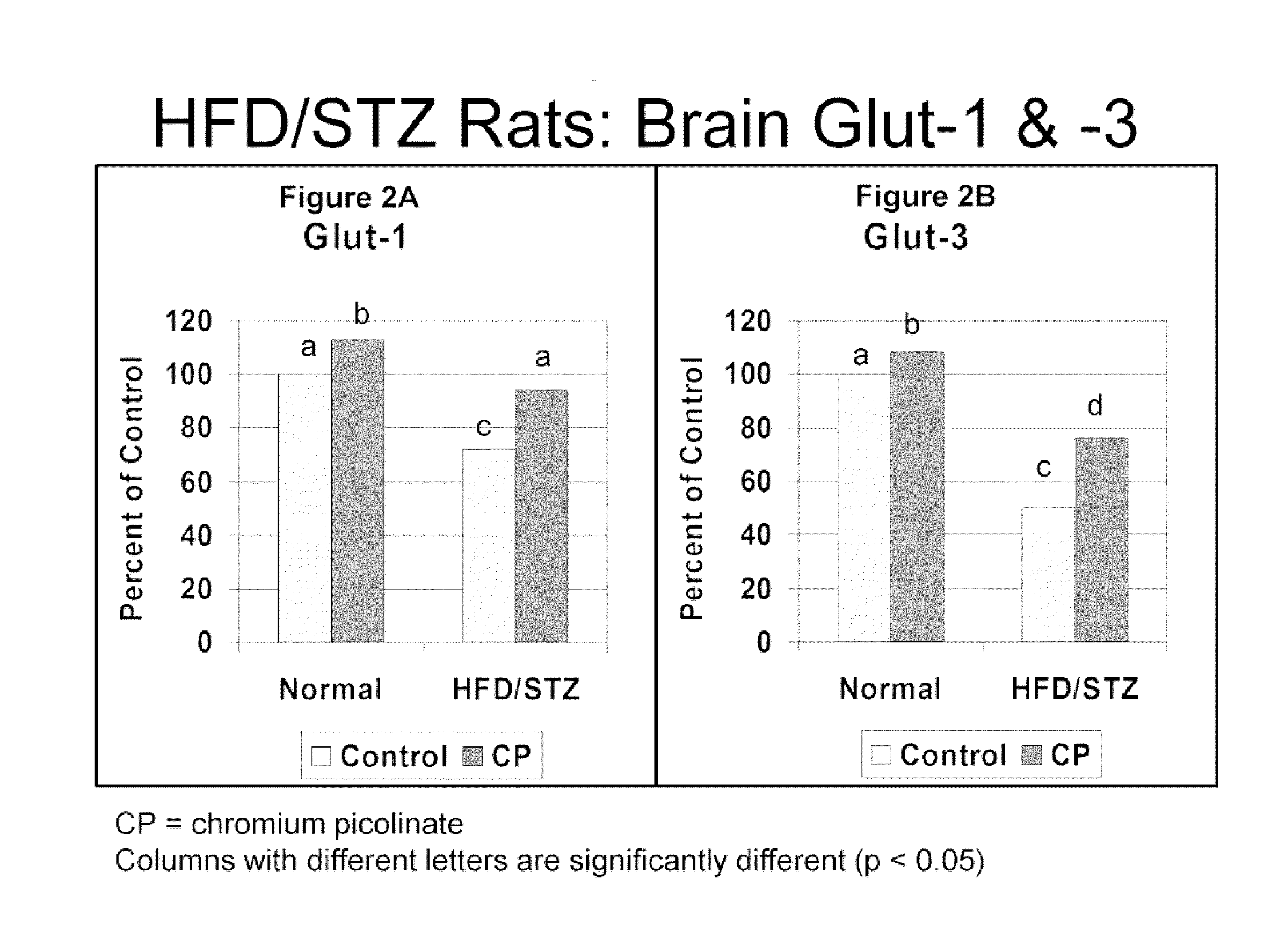
[0104]In order to test whether chromium affected diabetes-induced decreases in brain glucose transporter GLUT1 and GLUT3 levels, the levels of GLUT1 and GLUT3 expression products were compared between healthy control animals and animals with type 2 diabetes; with and without administration of chromium.
[0105]An animal model of type 2 diabetes was produced by feeding male Sprague-Dawley rats (n=10) with a high-fat diet (HFD, 40% Kcal from fat) for 2 weeks, then intraperitoneally administering streptozotocin (STZ, 40 mg / kg). The HFD-treated rats and the HFD / STZ-treated rats were then administered 80 μg chromium picolinate / kg body weight / day for 12 weeks. Chromium picolinate was obtained from Nutrition 21, Purchase, N.Y. Untreated Sprague-Dawley (standard control diet) and HFD / STZ-treated rats not administered chromium served as controls.
[0106]After 12 weeks, chromium levels and GLUT-1...
example 3
[0108]A subject is identified as having GLUT1 deficiency syndrome. The subject presents with one or more symptoms associated with GLUT1 deficiency syndrome such as the presence of a SLC2A1 mutation, neurological problems associated with GLUT1 deficiency such as stiffness caused by abnormal tensing of the muscles (spasticity), difficulty in coordinating movements (ataxia), and speech difficulties (dysarthria). The subject can experience episodes of confusion, lack of energy (lethargy), headaches, muscle twitches (myoclonus), or involuntary irregular eye movements, particularly before meals.
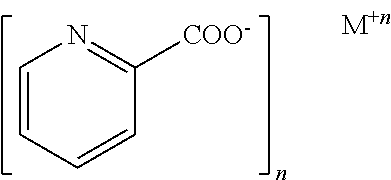
[0109]The subject is administered between 50 μg and 5000 μg chromium histidinate, chromium picolinate, or chromium histidinate / chromium picolinate combination complex / day. The chromium histidinate is administered orally. After a period of time, a reduction in one or more of the symptoms of GLUT1 deficiency is observed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com