Bone Augmentation Utilizing Muscle-Derived Progenitor Compositions in Biocompatible Matrix, and Treatments Thereof
a technology of biocompatible matrix and progenitor cells, applied in the field of muscle-derived progenitor cells, can solve the problems of low survival rate of cells following transplantation, association of primary myoblast-derived treatments, and polymer solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
MDC Enrichment, Isolation and Analysis According to the Pre-Plating Method
[0075]MDCs were prepared as described (U.S. Pat. No. 6,866,842 of Chancellor et al.). Muscle explants were obtained from the hind limbs of a number of sources, namely from 3-week-old mdx (dystrophic) mice (C57BL / 10ScSn mdx / mdx, Jackson Laboratories), 4-6 week-old norrnal female SD (Sprague Dawley) rats, or SCID (severe combined immunodeficiency) mice. The muscle tissue from each of the animal sources was dissected to remove any bones and minced into a slurry. The slurry was then digested by 1 hour serial incubations with 0.2% type XI collagenase, dispase (grade II, 240 unit), and 0.1% trypsin at 37° C. The resulting cell suspension was passed through 18, 20, and 22 gauge needles and centrifuged at 3000 rpm for 5 minutes. Subsequently, cells were suspended in growth medium (DMEM supplemented with 10% fetal bovine serum, 10% horse serum, 0.5% chick embryo extract, and 2% penicillin / streptomycin). Cells were then...
example 2
MDC Enrichment, Isolation and Analysis According to the Single Plate Method
[0079]Populations of rapidly- and slowly-adhering MDCs were isolated from skeletal muscle of a mammalian subject. The subject may be a human, rat, dog or other mammal. Biopsy size ranged from 42 to 247 mg.
[0080]Skeletal muscle biopsy tissue is immediately placed in cold hypothermic medium (HYPOTHERMOSOL® (BioLife) supplemented with gentamicin sulfate (100 ng / ml, Roche)) and stored at 4° C. After 3 to 7 days, biopsy tissue is removed from storage and production is initiated. Any connective or non-muscle tissue is dissected from the biopsy sample. The remaining muscle tissue that is used for isolation is weighed. The tissue is minced in Hank's Balanced Salt Solution (HBSS), transferred to a conical tube, and centrifuged (2,500×g, 5 minutes). The pellet is then resuspended in a Digestion Enzyme solution (Liberase Blendzyme 4 (0.4-1.0 U / mL, Roche)). 2 mL of Digestion Enzyme solution is used per 100 mg of biopsy t...
example 3
Small Intestine Submucosa Alleviates the Repair of a Critical Size Calvarial Defect in Mice
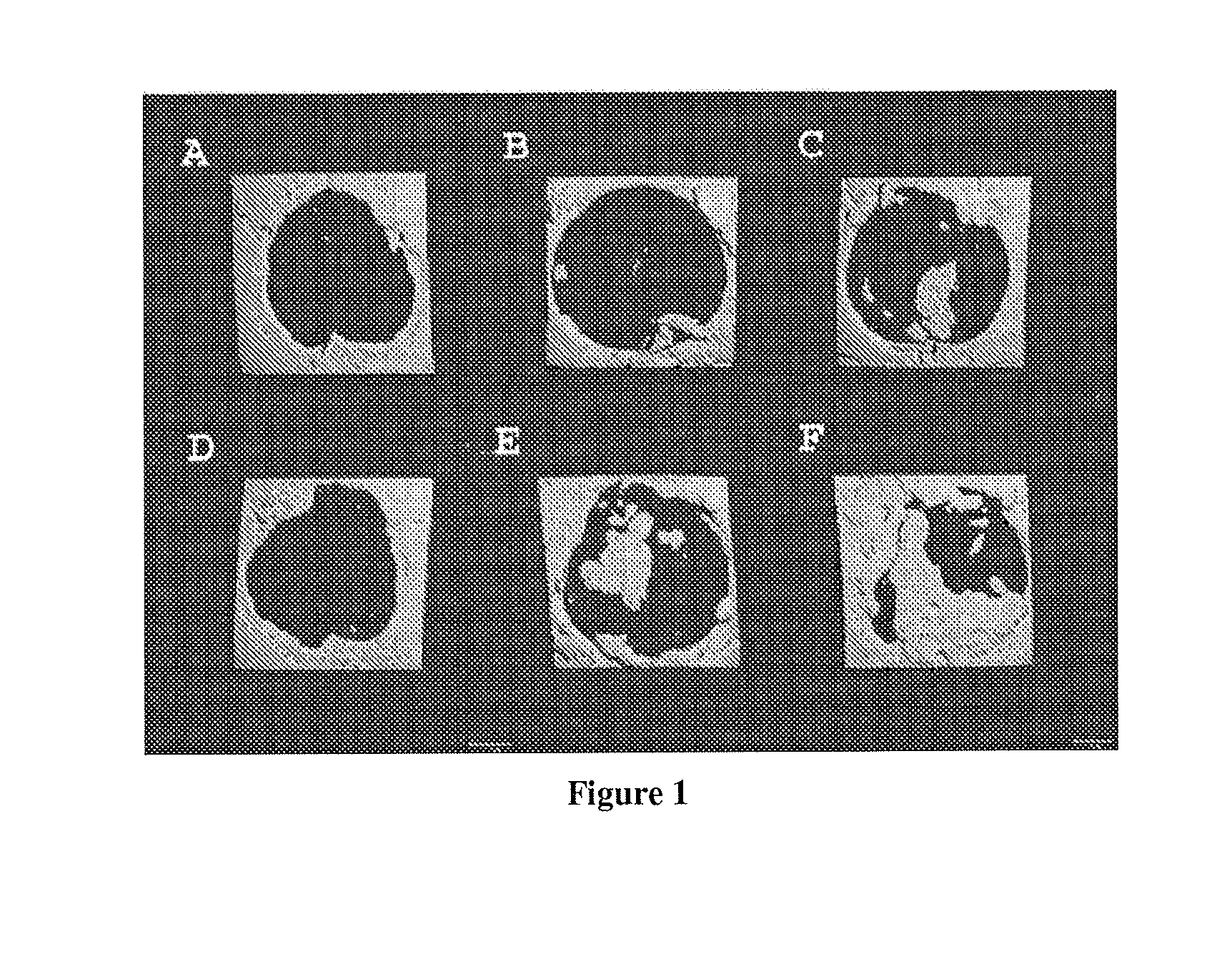
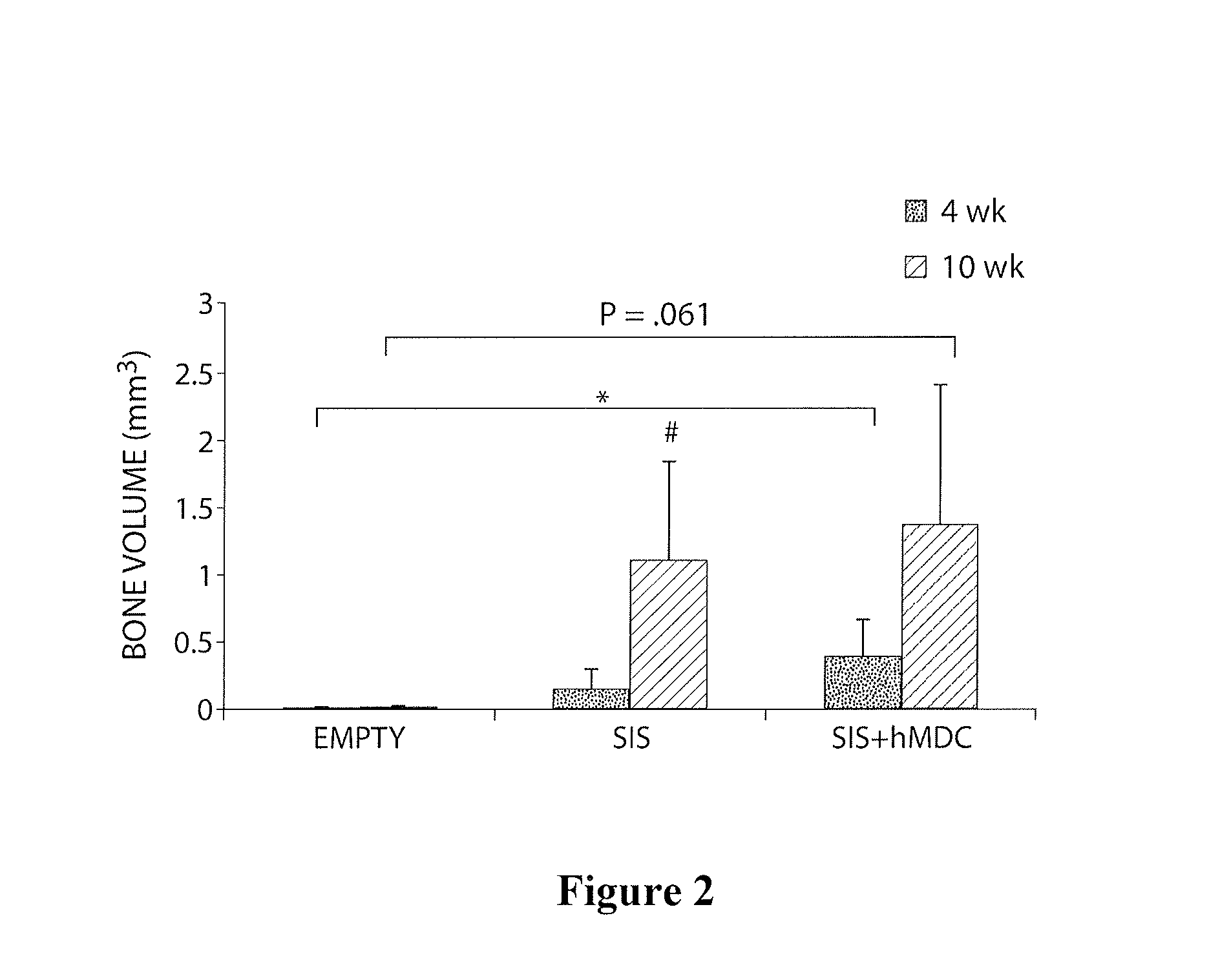
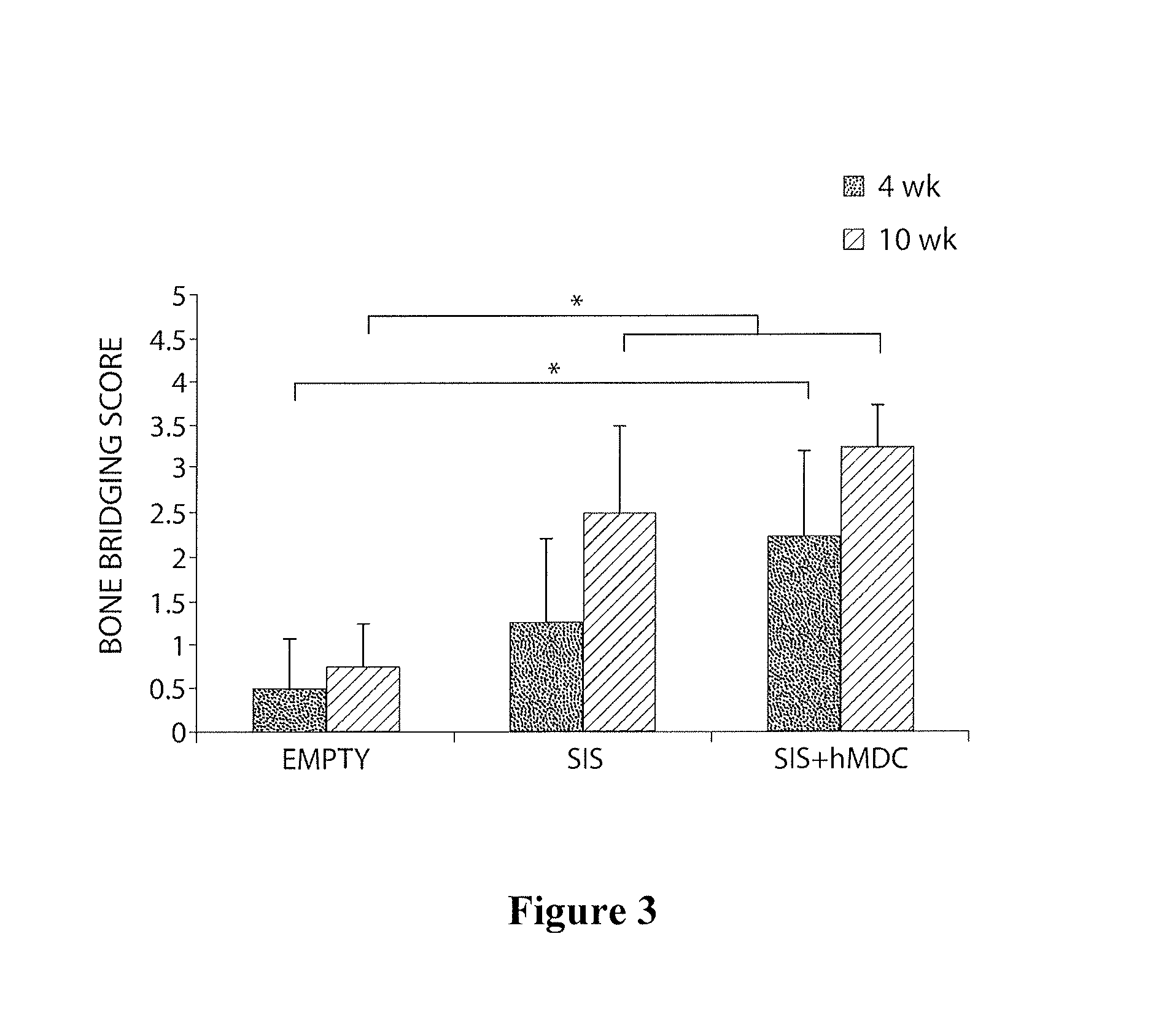
[0085]The purpose of this study was to investigate the bone regenerative potential of single-layer SIS scaffold transplanted into critical size calvarial defect in mice. We also preconditioned SIS grafts by seeding them with human muscle-derived cells (hMDCs), prepared as detailed in Example 2, above, in order to test osteogenic potential of this construct in response to natural fracture environment.
Materials and Methods
[0086]In this study a total of 24 SCID mice were used. All animal experiments were approved by institutional ARCC. Surgical procedure was performed under general anesthesia. Critical size calvarial bone defect was created using a 5-mm-diameter trephine burr. Human muscle-derived cells (hMDCs) isolated from a 35 year old male patient were provided. Animals were divided into 3 groups according to the treatment they received. A control group consisted of untreated mice with a calv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com