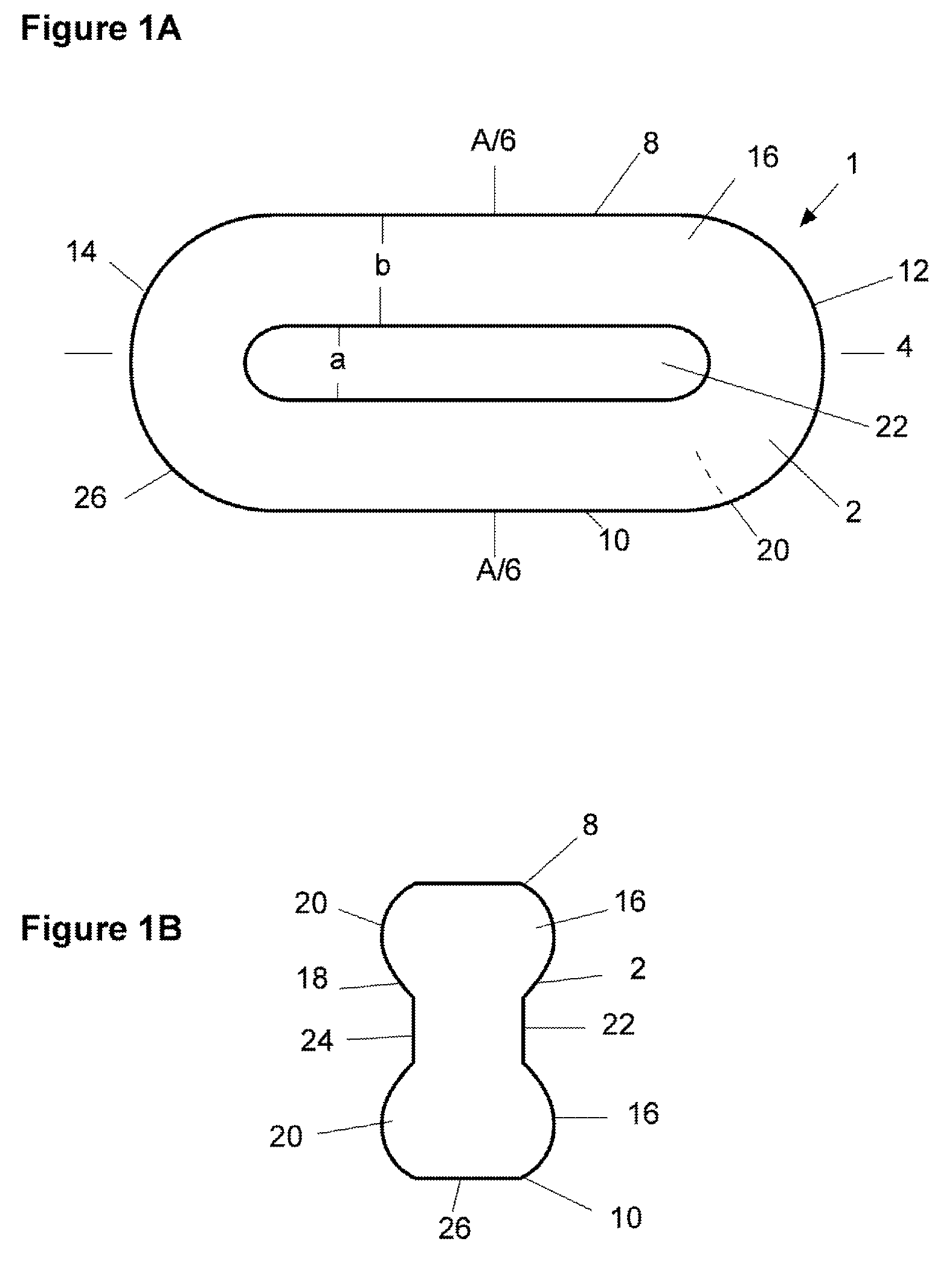
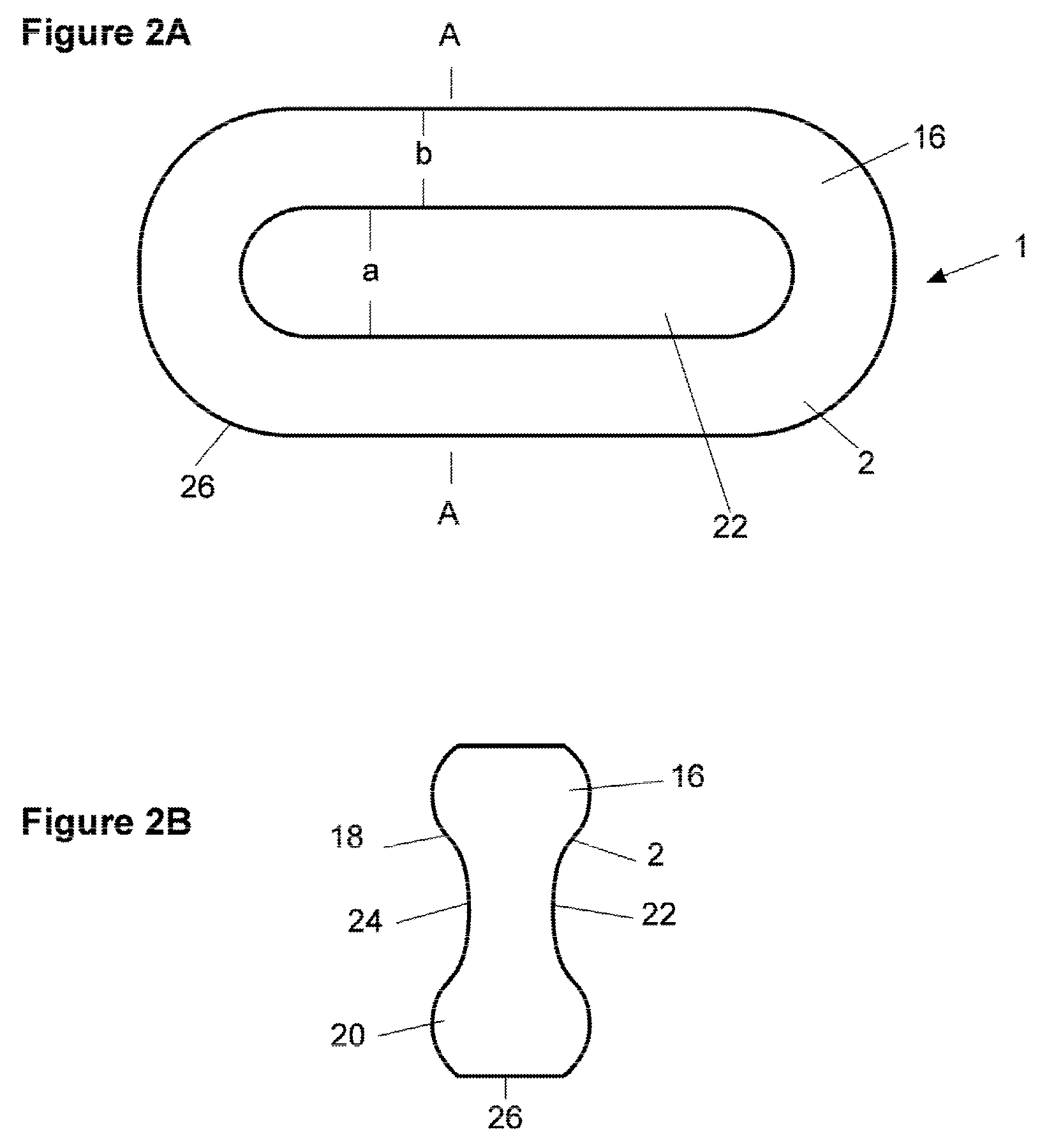
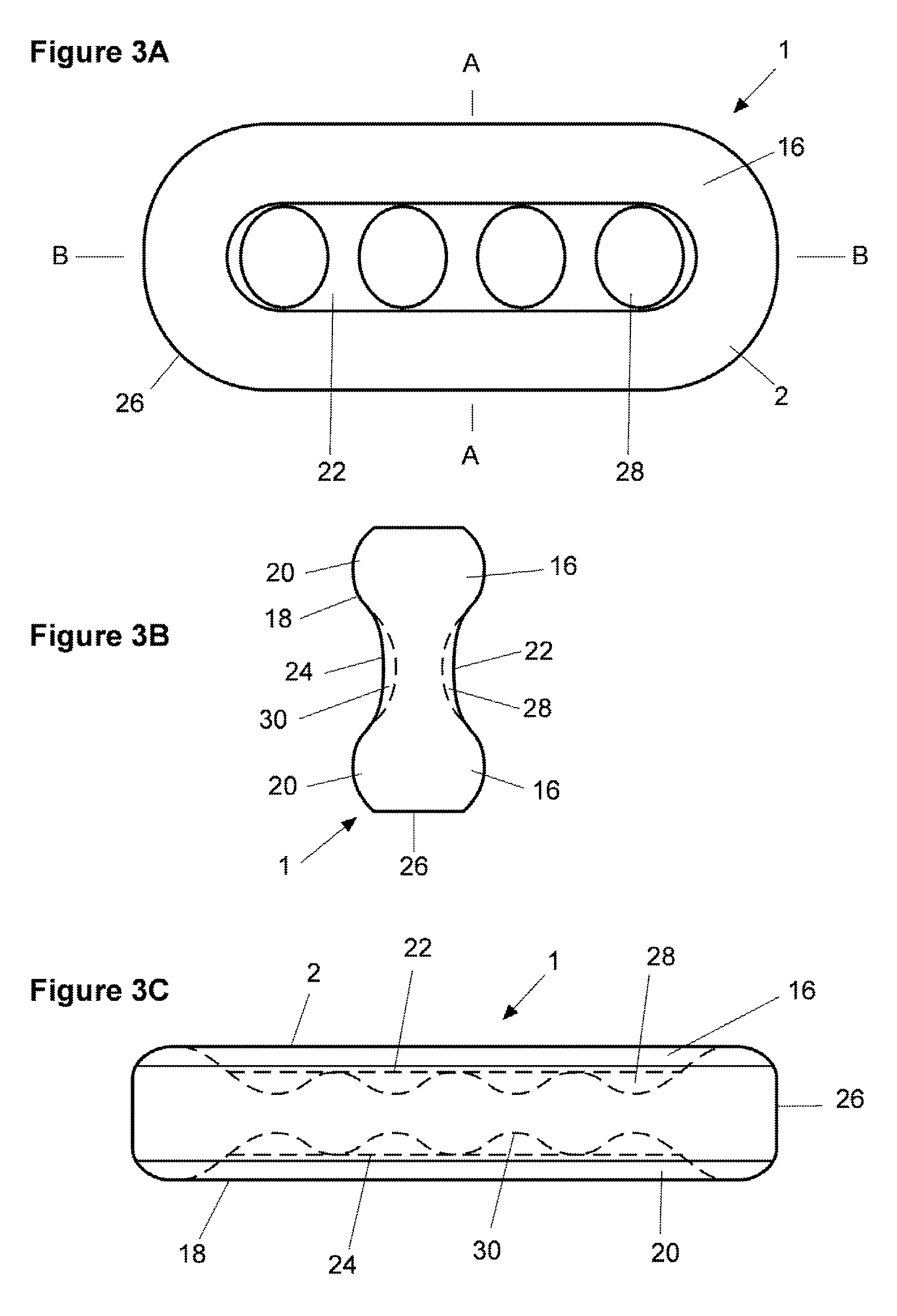
Pharmaceutical dosage form
a technology dosage forms, applied in the direction of drug compositions, nervous disorders, transportation and packaging, etc., can solve the problems of serious adverse effects or even death, particularly unpleasant, and has indeed proved rather unsatisfactory, and achieves fine adjustment or tailoring, accurate, predictable and reliable manner, and greater control of the release profile of pharmaceutically active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation Examples
A. Formulation Examples for Retarded Release Formulations
[0545]
Active ingredient(s)0.01-50%(w / w)Viscous hydrophilic polymer(s)0.01-80%(w / w)Pregelatinized starch5-80%(w / w)Pharmaceutically acceptable formulating agentsad 100%(w / w).Active ingredient(s)0.01-50%(w / w)Viscous hydrophilic polymer(s) comprising0.01-80%(w / w)hydroxypropyl cellulosePregelatinized starch5-80%(w / w)Pharmaceutically acceptable formulating agentsad 100%(w / w).Active ingredient(s)0.01-50%(w / w)Viscous hydrophilic polymer(s)0.01-80%(w / w)Pregelatinized starch5-15%(w / w)Pharmaceutically acceptable formulating agentsad 100%(w / w).Active ingredient(s)0.01-50%(w / w)Viscous hydrophilic polymer(s) comprising0.01-80%(w / w)hydroxypropyl cellulosePregelatinized starch5-15%(w / w)Pharmaceutically acceptable formulating agentsad 100%(w / w).Active ingredient(s)0.01-50%(w / w)Viscous hydrophilic polymer(s)0.01-80%(w / w)Pregelatinized starch5%(w / w)Pharmaceutically acceptable formulating agentsad 100%(w / w).Active ingredient(s...
example 2
[0566]Pharmaceutical dosage forms were manufactured from the following compositions:
I-1 (#1)I-2 (#2)C-1tapentadol HCl291.20291.20291.20PEO Mw 7 Mio g / mol245.00245.00PEO Mw 5 Mio g / mol247.70polyethylene glycole 600065.1065.1065.00HPMC 100,000 mPas98.0098.0045.50α-tocopherole0.700.700.65SUM700.00700.00650.00tablet format9 × 21 H09 × 21 H09 × 21 oblong291.20 mg tapentadol HCl correspond to 250 mg tapentadol free base.
General Procedure:
[0567]The polyethylene oxide was melted at 90° C. and the total amount of α-tocopherol was dissolved therein. Then, tapentadol and hydroxypropyl methyl cellulose (HPMC) were mixed in a fast mixer for 5 min at a temperature of 70° C. The melt of polyethylene oxide and α-tocopherole was added to the mixture dropwise within 10 minutes. The thus obtained granulate was sieved on a taper sieving machine and then mixed in a free fall mixer for 15 min. The powder mixture was dosed gravimetrically to an extruder. Extrusion was performed by means of a twin screw ex...
example 3
[0573]The dissolution profile of the tablets was investigated under the following conditions: Paddle apparatus equipped with sinker, 50 rpm, 37±5° C., 900 mL simulated intestinal fluid pH 6.8 (phosphate buffer).
[0574]The results are displayed in FIGS. 21 to 24.
[0575]FIG. 21 shows that immediately after manufacture the release profile of the tablet according to inventive example I-2 (H-shape) is comparable to the release profile of the tablet according to comparative example C-1 (oblong).
[0576]FIG. 22 shows that the release profile of the tablet according to inventive example I-1 (H-shape) is comparable to the release profile of the tablet according to inventive example I-2 (H-shape), i.e. that different batches provide reproducible results.
[0577]FIG. 23 shows that the release profile of the tablet according to comparative example C-1 (oblong) changes upon storage (40° C., 6 months; 25° C. 9 months; and 30° C. 9 months, respectively).
[0578]FIG. 24 shows that the release profile of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com