Sustained-release solid preparation for oral use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
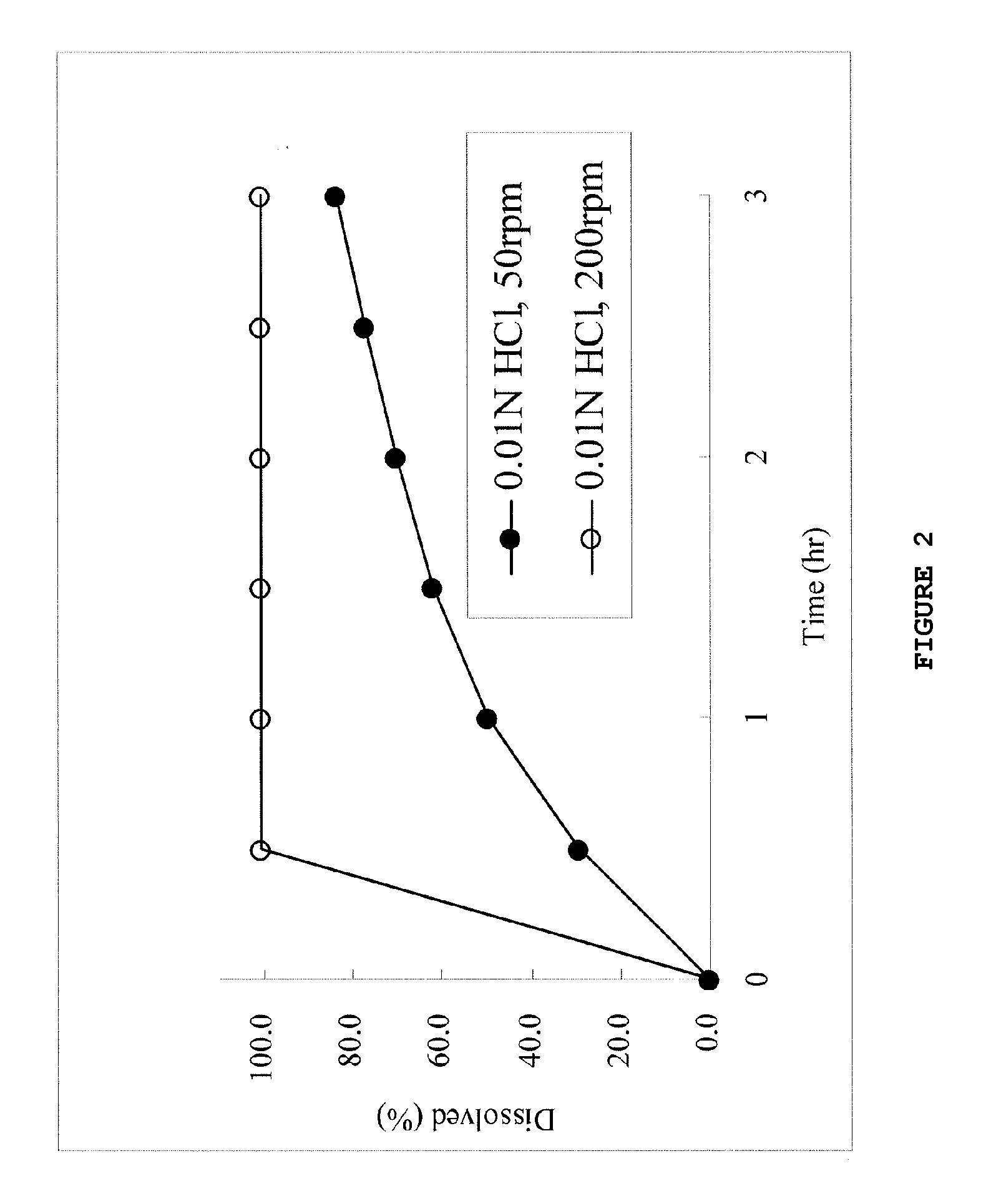
[0150]Tablets having formulations 1 and 1a shown in Table 1 were produced by mixing of each component using a mortar followed by the direct compression method and subjected to the dissolution test in an acidic or neutral solution. The results obtained from the acidic solution are shown in Table 2 and FIGS. 1 and 2. The results obtained from the neutral solution are shown in FIGS. 3 and 4.
TABLE 1Content (mg)Formulation 1Formulation 1aCompound (1a)36.436.4HPC-M fine60.060.0HPC-SL regular12.012.0HPMCAS-LF120.0—Mannitol59.6179.6Sodium stearyl fumarate12.012.0Total300.0300.0
TABLE 2Influence of paddle rotation rate in acidic test medium ontablets having formulations 1 and 1aFormulation 1Formulation 1aD2 h,200 rpm-D2 h,50 rpm3.6%30.6%D2 h,200 rpm / D2 h,50 rpm1.21.4
[0151]As is evident from FIGS. 3 and 4, both the tablets of formulations 1 and 1a exhibited prolonged dissolution properties in the neutral solution. On the other hand, it was demonstrated that the tablets of formulation 1a were l...
example 2
[0152]Tablets having formulations 1 and 2a shown in Table 3 were produced by mixing of each component using a mortar followed by the direct compression method and subjected to the dissolution test in an acidic solution. The results are shown in Table 4 and FIGS. 1 and 5.
TABLE 3Content (mg)Formulation 1Formulation 2aCompound (1a)36.436.4HPC-M fine60.060.0HPC-SL regular12.012.0HPMCAS-LF120.0—HPMCAS-LG—120.0Mannitol59.659.6Sodium stearyl fumarate12.012.0Total300.0300.0
TABLE 4Influence of paddle rotation rate in acidic test mediumon tablets having formulations 1 and 2aFormulation 1Formulation 2aD2 h,200 rpm-D2 h,50 rpm (%)4.315.0D2 h,200 rpm / D2 h,50 rpm1.21.6
[0153]The tablets of formulation 1 in which HPMCAS having a small particle size was used were less influenced by the paddle rotation rate in the acidic solution, than the tablets of formulation 2a in which HPMCAS having a large particle size was used. Thus, HPMCAS having a small particle size was effective for maintenance of tablet ...
example 3
[0154]Tablets having formulations 1 and 3a shown in Table 5 were produced by mixing of each component using a mortar followed by the direct compression method and subjected to the dissolution test in a neutral solution, a dissolution test using USP Apparatus 3, and in vivo absorption property evaluation using dogs. The results of the dissolution test in the neutral solution are shown in FIG. 6.
TABLE 5Content (mg)Formulation 1Formulation 3aCompound (1a)36.436.4HPC-M fine60.060.0HPC-SL regular12.012.0HPMCAS-LF120.0120.0Mannitol59.6—Microcrystalline cellulosea)—59.6Sodium stearyl fumarate12.012.0Total300.0300.0a)grade PH101
[0155]As shown in FIG. 6, the tablets obtained using mannitol or crystalline cellulose exhibited prolonged drug dissolution in the neutral solution. On the other hand, the dissolution test using USP Apparatus 3 showed that the tablets of formulation 1 in which mannitol was used exhibited prolonged drug dissolution, whereas the tablets of formulation 3a in which cryst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com