Cationic polymer coated mesoporous silica nanoparticles and uses thereof
a technology of mesoporous silica and nanoparticles, which is applied in the field of submicron structures, can solve the problems of encapsulated drugs being lost from the carrier or degraded, overblown claims, and agglomeration in the circulation, so as to reduce the translation of p-glycoproteins, and reduce the translation of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis and Surface Modification of Mesoporous Silica Nanoparticles MSNP
[0236]The basic synthesis of MSNP was conducted by mixing the silicate source tetraethylorthosilicate (TEOS) with the templating surfactants cetyltrimethylammonium bromide (CTAB) in basic aqueous solution (pH 11). In a round bottom flask, 100 mg CTAB was dissolved in a round-bottom flask containing solution of 48 ml distilled water and 1.75 ml sodium hydroxide (2 M). The solution was heated to 80° C. and stirred vigorously. After the temperature had stabilized, 0.5 ml TEOS was added slowly into the heated CTAB solution. After 15 min, 0.23 mmol of the organosilane solution was added into the mixture. 3-trihydroxysilylpropyl methylphosphonate was used for phosphonate surface modification, and aminopropyltriethoxy silane (APTS) was used for amine surface modification. After 2 hr, the solution was cooled to room temperature and the materials were washed with methanol using centrifugation. In order to incorporate f...
example 2
Physicochemical Characterization of the NP
[0239]MSNP were synthesized according to a modified procedure previously described (Radu et al., J. Am. Chem. Soc., vol. 126, pp. 13216-13217, 2004; Cai et al., Chem. Mater., vol. 13, no. 2, pp. 258-263, 2001). All MSNP were characterized for size, size distribution, shape, and charge (Table 1).
TABLE 1Size distribution of MSNPs in aqueous solutions.ZetaSize (nm)Potential (mV)DMEMBEGMH2O / DMEM +10%2 mg / mlserum / H2OserumBEGMBSABEGM + BSAMSNP-OH19664081096416−10.5 / −6.8 / −7.2MSNP-Phosphate1975306867439−8.9 / −6.5 / −5.8MSNP-PEG26754051215542−10.4 / −5.9 / −4.5MSNP-PEI 0.616894151243474+29.5 / −7.8 / −6.5KDMSNP-PEI 1.216844521298502+38.7 / −6.5 / −7.1KDMSNP-PEI 1.810535101087550+36.9 / −5.4 / −3.2KDMSNP-PEI 10614702917684+34.1 / −7.5 / −6.9KDMSNP-PEI 25147310431544825+30.8 / −5.9 / −4.0KDParticle size and zeta potential in solution were measured by a ZetaSizer Nano (Malvern).DMEM = Complete Dulbecco's Modified Eagle Media, which contains 10% fetal calf serum (FCS).BEGM = Bronc...
example 3
Differences in the Cytotoxic Potential of Anionic Vs Cationic MSNP
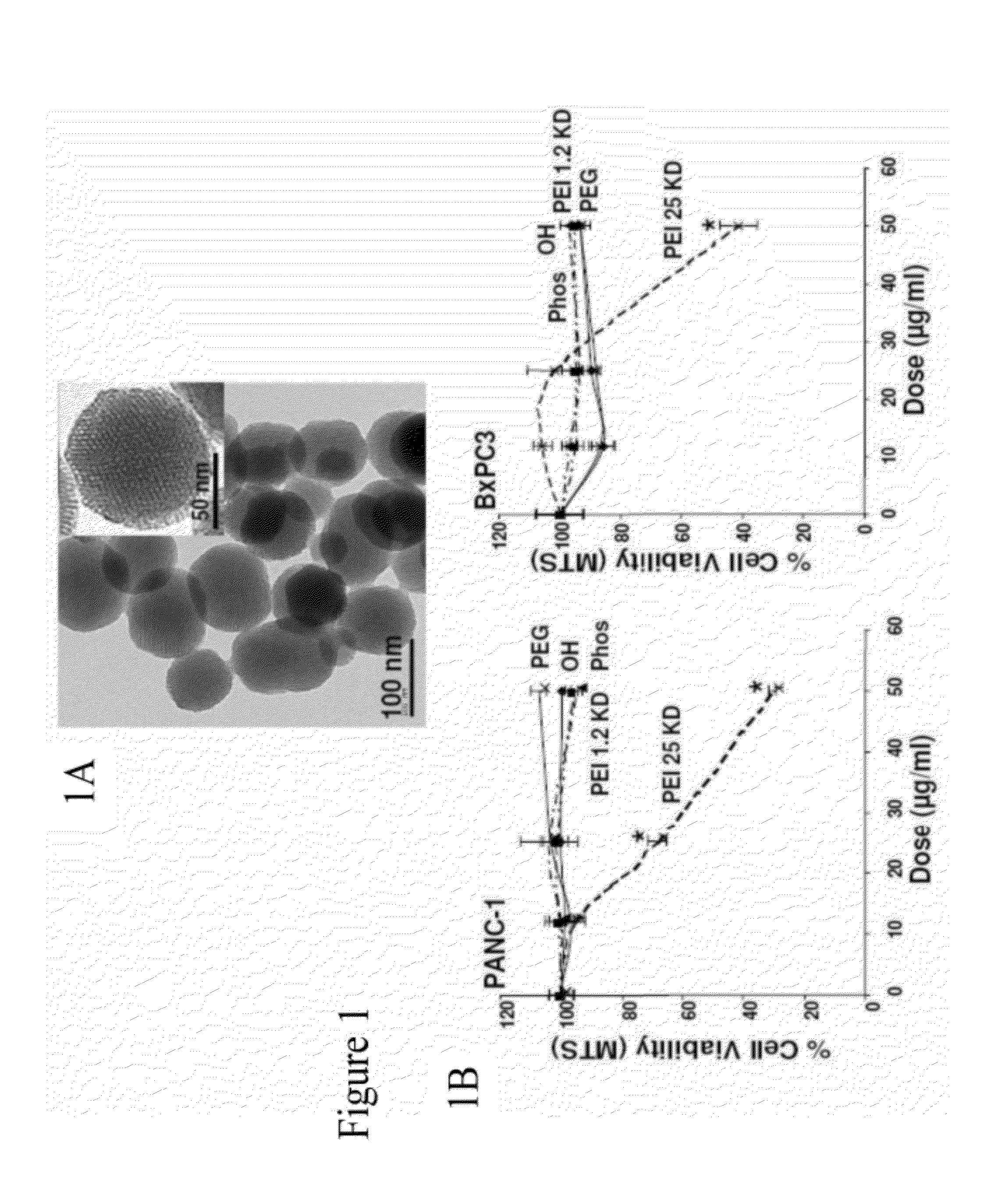
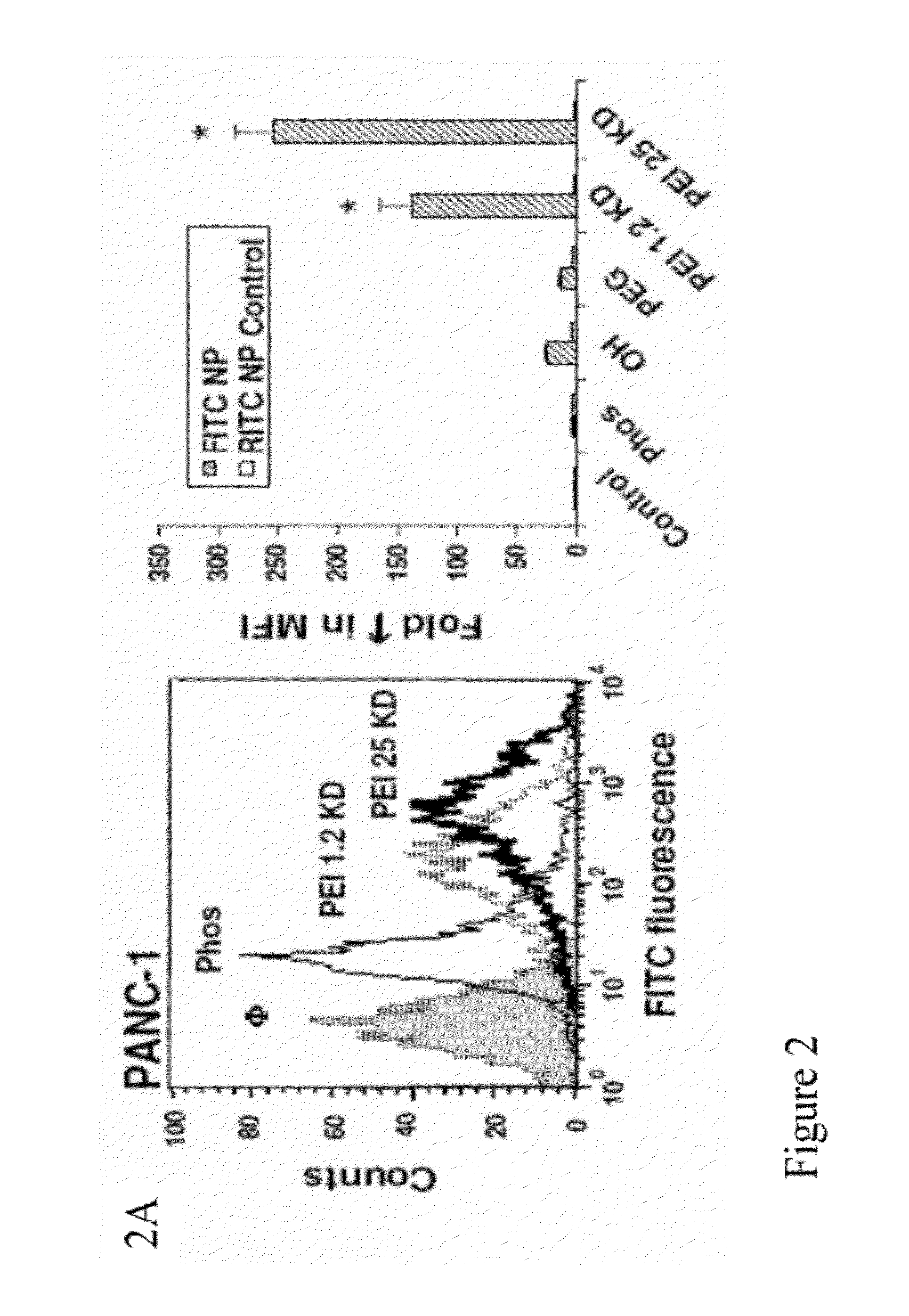
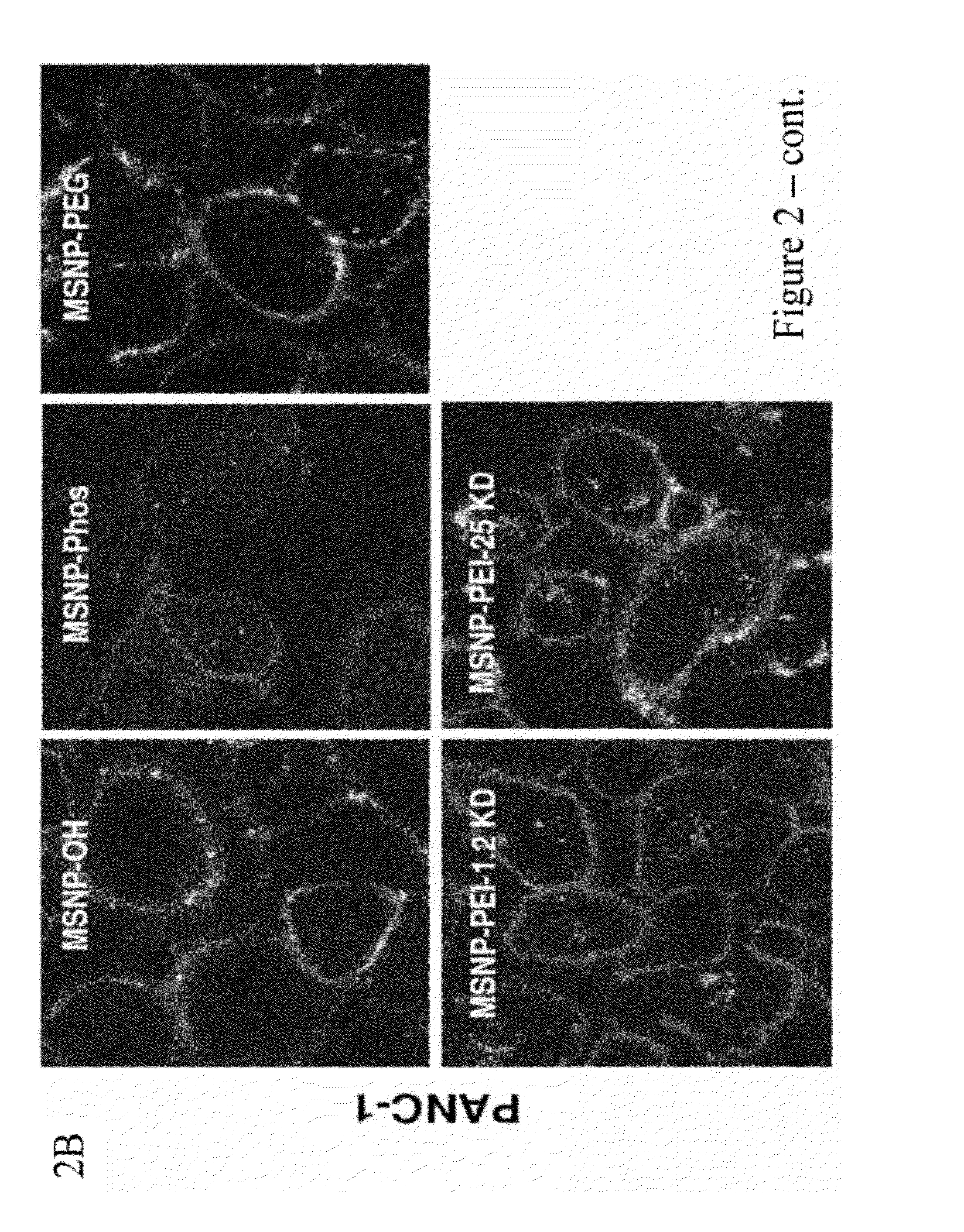
[0242]To screen for particle hazard, the MTS assay was used, which reflects dehydrogenase activity in healthy cells (Xia et al., ACS Nano, vol. 2, pp. 85-96, 2008). While most of the MSNP did not interfere in MTS activity in the PANC-1 and BxPC3 pancreatic cancer cell lines, particles coated with the 25 KD PEI polymer showed decreased cellular viability (FIG. 1). The particles coated with the 25 KD polymer also induced toxicity in macrophage (RAW 264.7) and bronchial epithelial (BEAS-2B) cell lines staining (FIG. 10). The toxicity was confirmed by propidium iodide (PI) staining, which showed that the rate of cell death was progressive over 15 hour period (FIG. 10). Similar to previous results with cationic polystyrene nanoparticles (Xia et al., ACS Nano, vol. 2, pp. 85-96, 2008), the toxicity of cationic MSNP involves an effect on mitochondria as determined by JC-1 fluorescence (FIG. 10). JC-1 measures mitochondrial m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ensemble average diameter | aaaaa | aaaaa |
| ensemble average diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com