Quickly soluble oral film dosage containing steviosides as a unpleasant taste masking agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 14
[0093]Ondansetron based quickly soluble film prepared according to Example 9 of the present invention was subjected to dissolution experiments at pH 1.2 in comparison with a conventional product named Zofran Zydis tablet containing ondansetron available from GlaxoSmithKline Co. (CSK) based on Notice of the Food and Drug Administration and the experimental results are shown in FIG. 1. As shown in the accompanying drawings, there was no substantial difference in elution between both of the formulations.
example 15
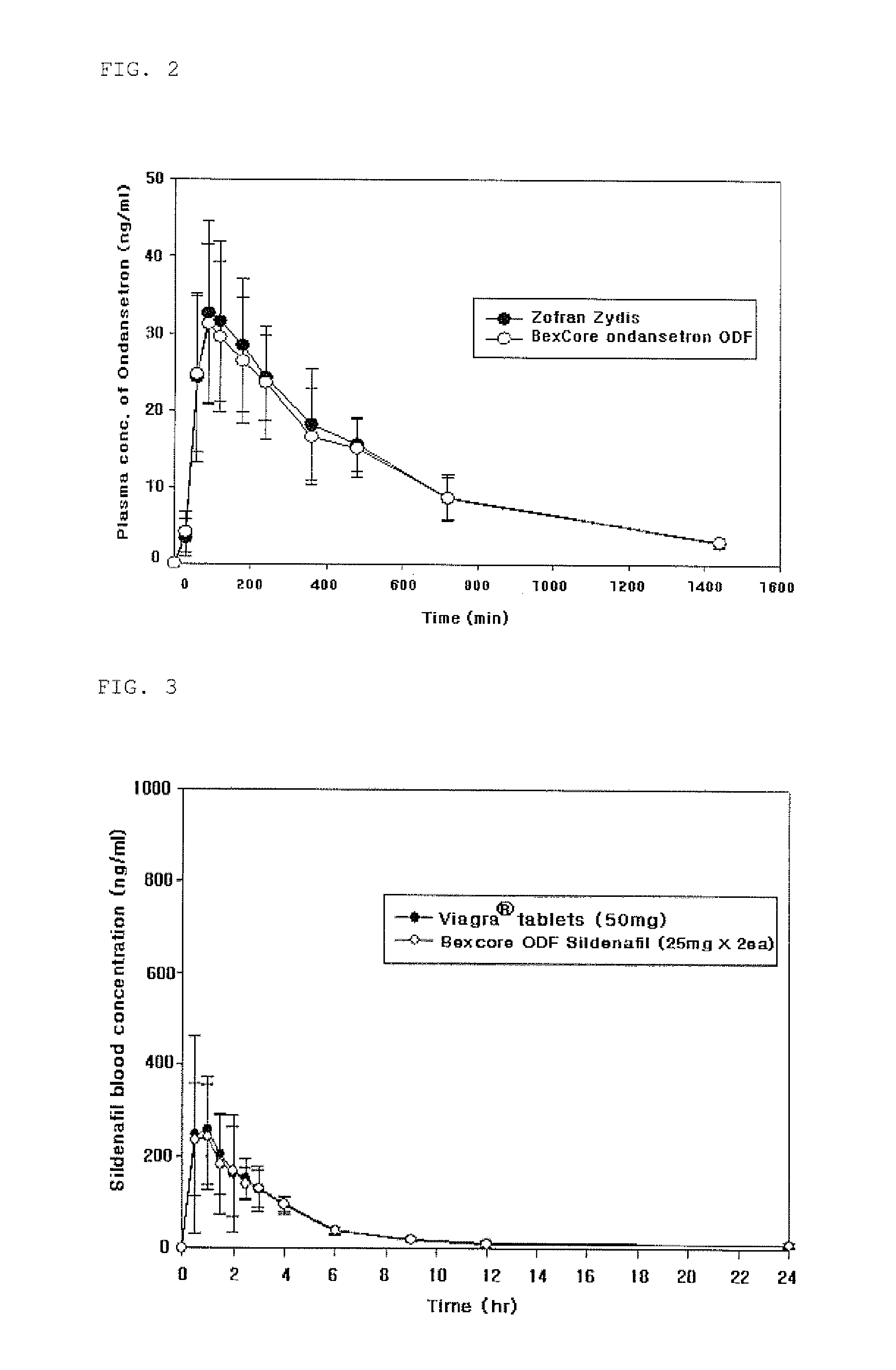
[0094]Ondansetron based quickly soluble film prepared according to Example 9 of the present invention was subjected to pharmacokinetic tests in comparison with a conventional product named Zofran® Zydis tablet containing ondansetron available from GlaxoSmithKline Co. (GSK). The experiments is conducted for healthy fourteen (14) adult men and women with Latin square method based on Notice of bioequivalence test standard of the Food and Drug Administration.
[0095]The experimental results are shown in Table 9 and FIG. 2. It was confirmed that the inventive film of this example has the bioequivalence as shown in Table 9 and FIG. 2.
TABLE 9Pharmacokinetic test result of ondansetron film formulationsParameterGroup 1Group 2AUCO~24(ng · hr / mL)244.78 ± 91.83 259.64 ± 87.10Cmax(ng / mL)30.93 ± 10.7133.16 ± 8.89Tmax(hr)1.86 ± 0.71 1.94 ± 0.75Note 1)Group 1 is Ondansetron film formulations (8 mg). Group 2 is Zorfran ®Zydis ODTs
example 16 to 20
[0096]As active pharmaceutical ingredients, sildenafil free base, sildenafil lactate, sildenafil citrate, granisetron, and montelukast sodium were added to prepare films having constitutional compositions shown in the following Table 10. The sensory test was randomly conducted and test results were that unpleasant aftertaste was well masked.
TABLE 10Exam-Exam-Exam-Exam-Exam-ple 15ple 16ple 17ple 18ple 19Ingredient(wt. %)(wt. %)(wt. %)(wt. %)(wt. %)Sucralose1.7221.50.5Neotame120Acesulfame0.50.510.5potassiumRebaten 97%22220.5Citric acid0.20.20.20.60.6Hydroxypropyl22232starchSpan200.10.50.10.40.4Menthol0.42.30.333Carageenan0.10.10.10.10.2Pigment0.10.10.10.10.1Polysorbate 800.40.50.30.30.3Peppermint oil0.82.70.633Pullulan62.377.555.181.578.9Microcrystalline1.41.40.71.54celluloseBeta0.7cyclodextrinLemon flavor220.81.51.5Sildenafil25free baseSildenafil4.2lactateSildenafil35citrateGranisetron1hydrochlorideMontelukast5sodiumTotal weight100.0100.0100.0100.0100.0
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com