Immunocompromised Ungulates
a technology of immunocompromised ungulates and ungulates, which is applied in the field of immunocompromised ungulates, can solve problems such as technical limitations, and achieve the effect of effective modeling human infectious disease pathologies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction and Design of Hc KO Vector
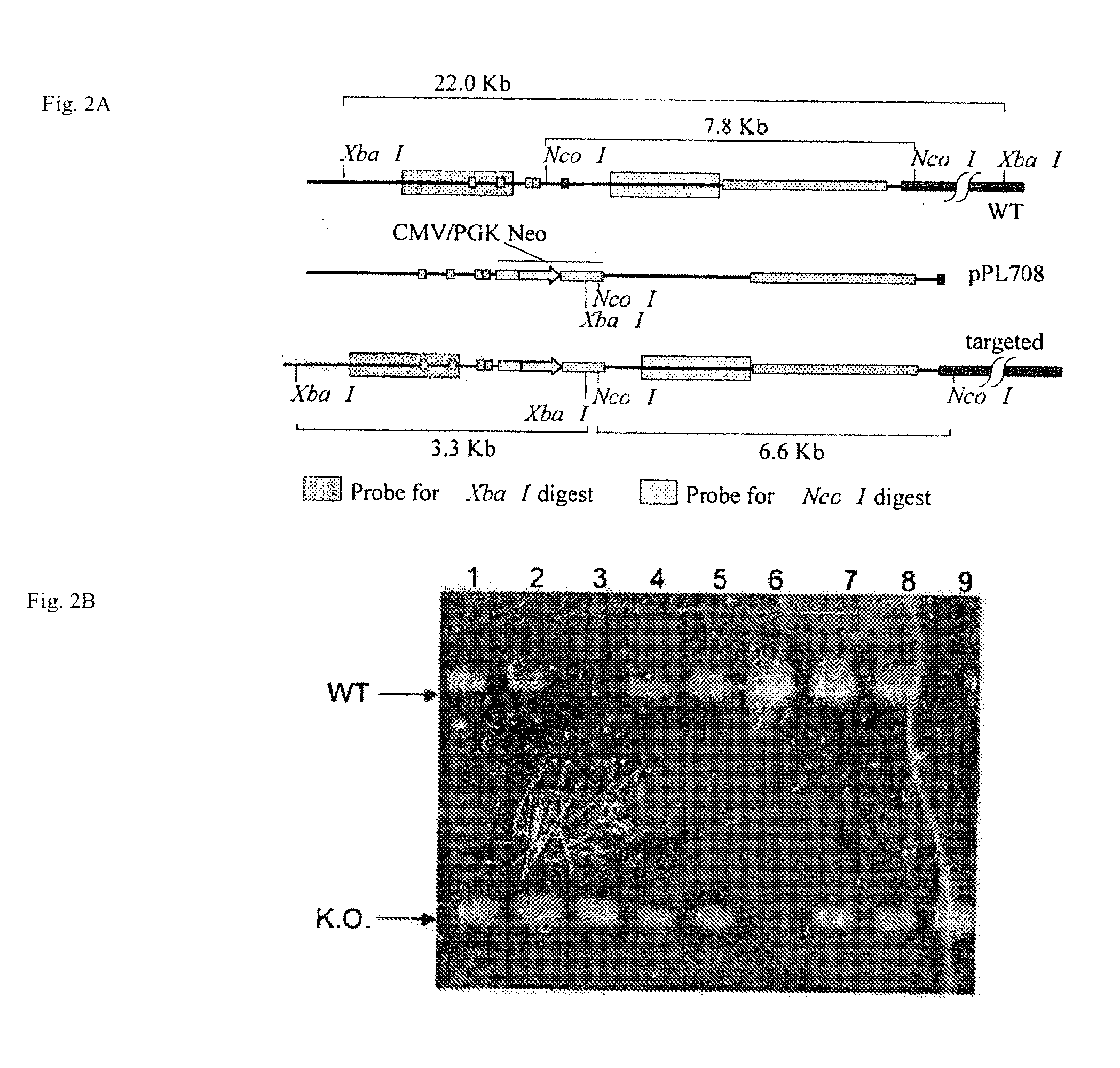
[0167]A portion of the genomic porcine Ig Hc locus was cloned and characterized. FIG. 1 illustrates the architecture of the porcine Hc locus. The single functional JH was found to reside within 6 kb upstream of the coding sequence for the Mu constant (Cμ region). The porcine JH was identified as an ‘Achilles heel’ that could be deleted to produce only non-functional VDJ rearrangement and, thus, prevent B-cell survival.
[0168]A poly (A) trap targeting vector was constructed (pPL708, FIG. 1) by flanking a CMV enhancer-pgk-neoR cassette that lacked polyA signal sequences, with a 2 kb upstream arm and a 5.8 kb downstream arm of homology. The downstream arm retained the complete JH to Cμ intron, and a few by of 3′ JH sequence, to insure normal splicing between the neoR transcript and the poly(A) signals found downstream of the Cμ coding region.
Vector Construction. Hc sequence data required for construction of a porcine Hc gene-targeting vector was ge...
example 2
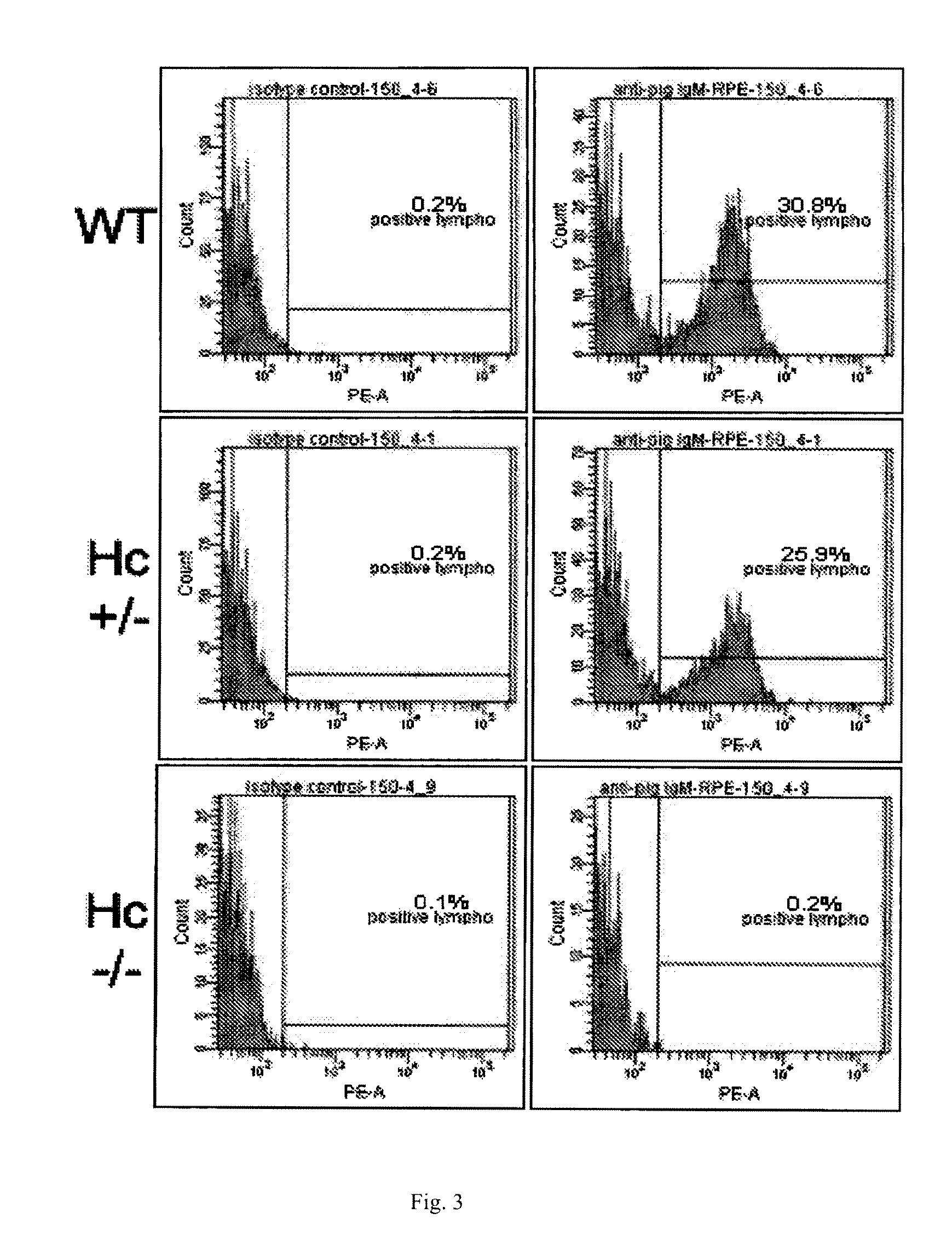
Heterozygous Hc KO in Porcine Cells
[0169]Isolation and transfection of primary porcine fetal fibroblasts. PCFF4-1 to PCFF4-10 PPFF cells were isolated from 10 fetuses of the same pregnancy at day 33 of gestation. After removing the head and viscera, fetuses were washed with Hanks' Balanced Salt Solution (HBSS, Invitrogen), placed in 20 ml of HBSS and diced with small surgical scissors. The tissue pellet was resuspended in 50 ml tubes with 40 ml of DMEM+100 U / ml collagenase (Invitrogen) per fetus. Tubes were incubated for 40 min in a shaking water bath at 37° C. The digested tissue was allowed to settle for 3-4 min and the cell-rich supernatant was transferred to a new 50 ml tube and spun down. The cells were then resuspended in 40 ml of DMEM, 10% Fetal Calf Serum, 1× non-essential amino acids, 1 mM sodium pyruvate (Invitrogen) and 2 ng / ml basic fibroblast growth factor (Roche Molecular Biochemicals, Indianapolis, Ind.), then seeded into 10 cm dishes. All cells were cryopreserved upo...
example 3
Somatic Cell Nuclear Transfer to Produce Heterozygous Hc KO Pigs
[0171]Somatic Cell Nuclear Transfer (SCNT) procedure. Cells from one of the three correctly targeted colonies obtained from transfection of pPL708 into female cell line PCFF4-8 were used as nuclear donors for SCNT. SCNT procedures were performed on in vitro matured oocytes (BoMed, Madison, Wis. and / or TransOVA, IA) using techniques described in the literature (Polejaeva, et al., (2000) Nature 407, 86-90, Dai et al., (2002) Nature biotechnology 20, 251-255, Campbell et al., (2007) Theriogenology 68 Suppl 1, S214-231, Vatja et al., (2007) Reprod Fertil Dev 19, 403-423). Electrical fusion and activation of reconstructed oocytes was performed using an ECM2001 Electrocell Manipulator (BTX Inc., San Diego). Fused nuclear transfer embryos were cultured in NCSU-23 medium for 1-4 h at 38.5° C., and then transferred to the oviduct of an estrus-synchronized recipient gilt. Crossbred gilts (large white / Duroc / Landrace) (280-400 lbs)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com