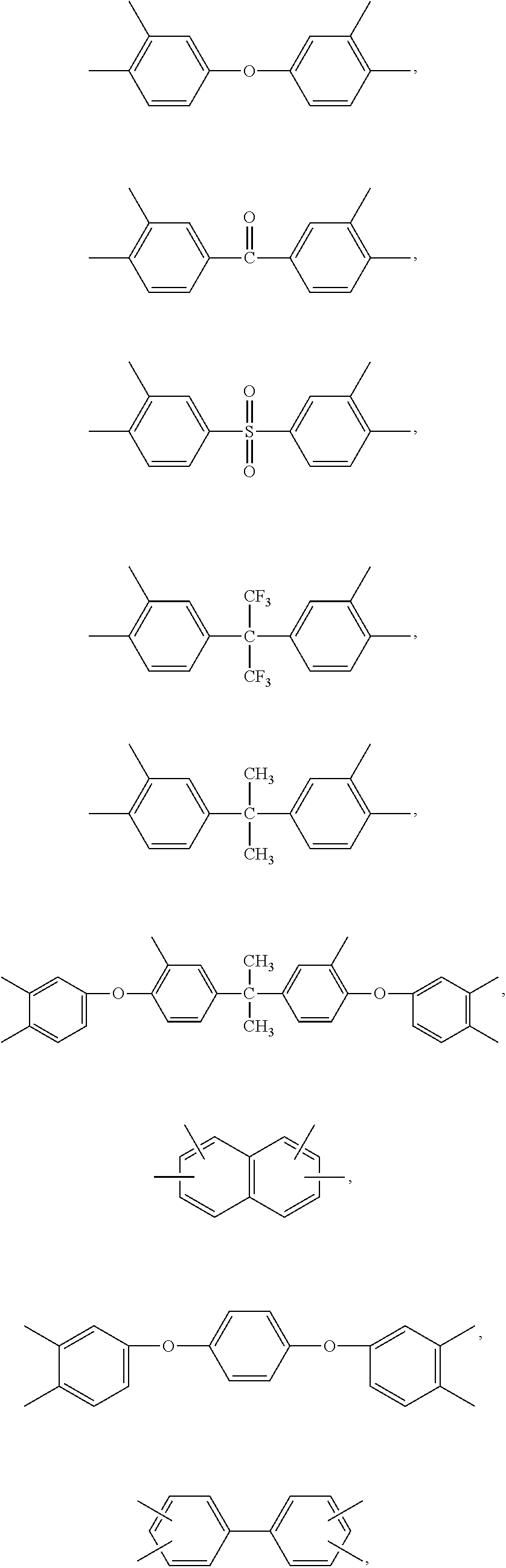
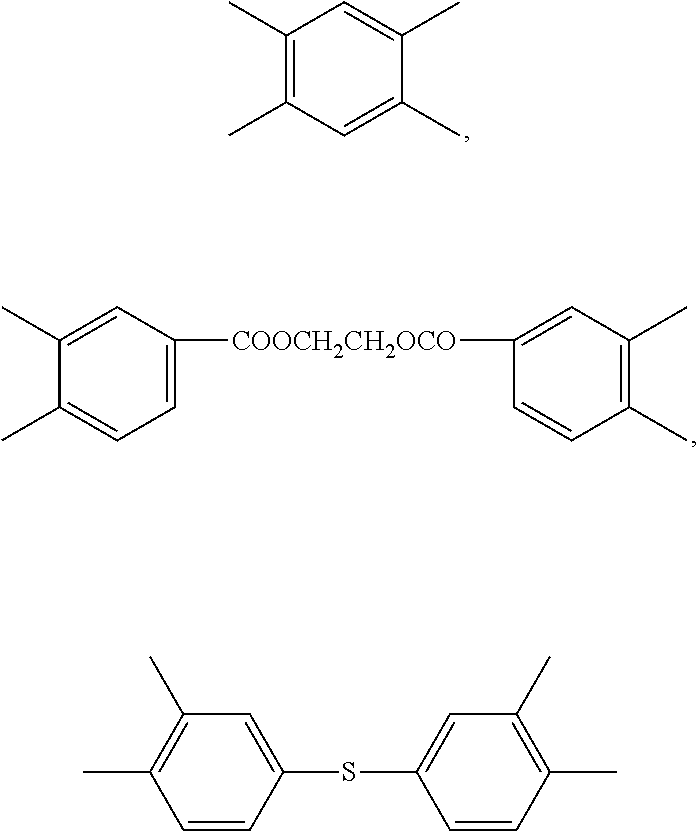
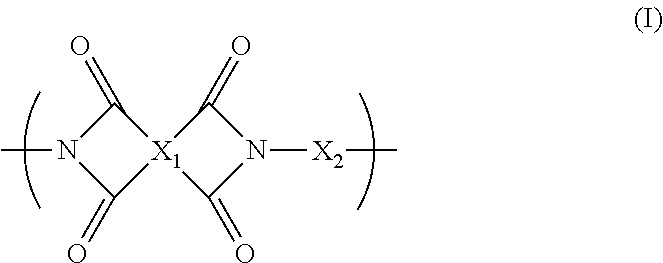Blend polymer membranes comprising thermally rearranged polymers derived from aromatic polyimides containing ortho-positioned functional groups
a polymer membrane and functional group technology, applied in the direction of isotope separation, inorganic chemistry, separation processes, etc., can solve the problems of thermal stability and contaminant resistance limitations of traditional polymer membranes, inability to meet the needs of the customer, etc., to achieve the effect of improving processability, reducing cost and improving flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of poly(6FDA-HAB) polyimide
[0060]The aromatic poly[2,2′-bis-(3,4-dicarboxyphenyl) hexafluoropropane dianhydride-3,3′-dihydroxy-4,4′-diamino-biphenyl] (poly(6FDA-HAB)) polyimide containing pendent —OH functional groups ortho to the heterocyclic imide nitrogen in the polymer backbone was synthesized from 2,2′-bis-(3,4-dicarboxyphenyl) hexafluoropropane dianhydride (6FDA) and 3,3′-dihydroxy-4,4′-diamino-biphenyl (HAB) in NMP polar solvent by a two-step process involving the formation of the poly(amic acid) followed by a solution imidization process. Acetic anhydride was used as the dehydrating agent and pyridine was used as the imidization catalyst for the solution imidization reaction. For example, a 250 mL three-neck round-bottom flask equipped with a nitrogen inlet and a mechanical stirrer was charged with 5.9 g (27.3 mmol) of HAB and 40 mL of NMP. Once HAB was fully dissolved, a solution of 6FDA (12.1 g, 27.3 mmol) in 40 ml, of NMP was added to the HAB solution in the fla...
example 2
Preparation of blend polymer membrane from poly(6FDA-HAB) polyimide and polyethersulfone (PES) (abbreviated as poly(6FDA-HAB) / PES)
[0061]The poly(6FDA-HAB) / PES blend polymer membrane was prepared as follows: 3.0 g of poly(6FDA-HAB) polyimide synthesized in Example 1 and 1.5 g of polyethersulfone (PES, purchased from BASF Corporation) were dissolved in a solvent mixture of 12.0 g of NMP and 12.0 g of 1,3-dioxolane. The mixture was mechanically stirred for 2 hours to form a homogeneous casting dope. The resulting homogeneous casting dope was allowed to degas overnight. The poly(6FDA-HAB) / PES blend polymer membrane was prepared from the bubble free casting dope on a clean glass plate using a doctor knife with a 20-mil gap. The membrane together with the glass plate was then put into a vacuum oven. The solvents were removed by slowly increasing the vacuum and the temperature of the vacuum oven. Finally, the membrane was dried at 200° C. under vacuum for at least 48 hours to completely re...
example 3
Preparation of polybenzoxazole / PES blend polymer membranes (PBO(6FDA-HAB) / PES-450C and PBO(6FDA-HAB) / PES-400C) from poly(6FDA-HAB) / PES blend polymer membrane by heat treatment
[0062]The poly(6FDA-HAB) / PES blend polymer membrane was thermally heated from 50° C. to 400° C. or 450° C. at a heating rate of 5° C. / min under N2 flow. The membrane was hold for 1 hour at 400° C. (to make PBO(6FDA-HAB) / PES-400C blend polymer membrane) or 450° C. (to make PBO(6FDA-HAB) / PES-450C blend polymer membrane) and then cooled down to 50° C. at a heating rate of 5° C. / min under N2 flow to yield polybenzoxazole / PES blend polymer membranes (PBO(6FDA-HAB) / PES-400C and PBO(6FDA-HAB) / PES-450C).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com