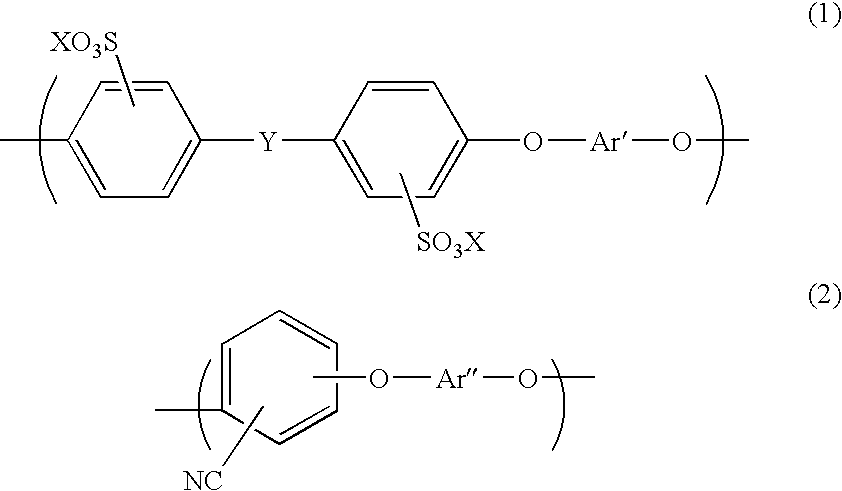
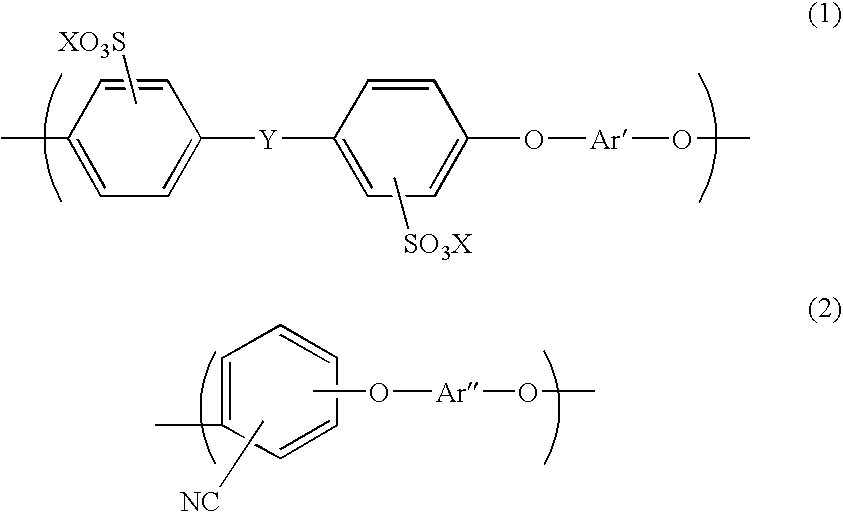
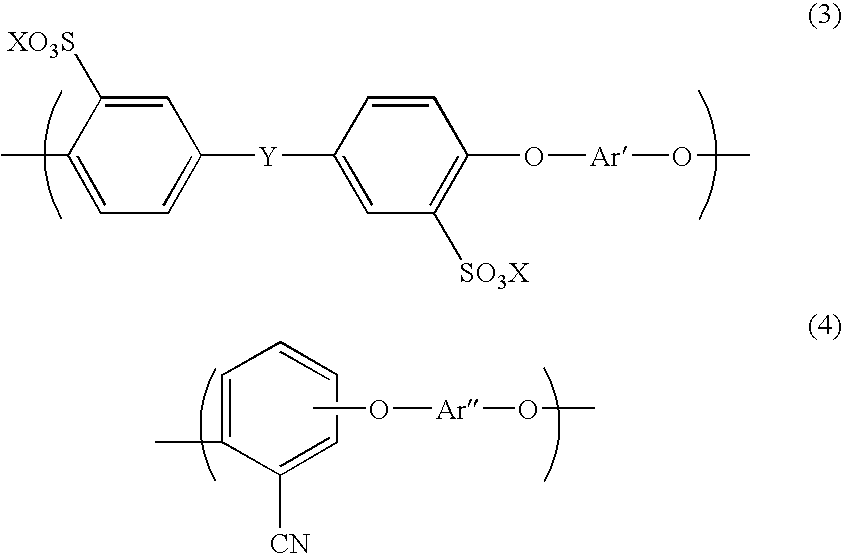
Aromatic hydrocarbon based proton exchange membrane and direct methanol fuel cell using same
a proton exchange membrane and hydrocarbon based technology, applied in the direction of fuel cell details, ion-exchangers, electrochemical generators, etc., can solve the problems of affecting the establishment of fuel cell techniques, affecting the performance of the cell as a whole, and excessively high membrane cost, so as to improve the energy density improve the efficiency of the fuel cell, and reduce the size of the fuel cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0082] Working examples of the invention will be described hereinafter, but the invention is not limited to the examples.
examples 1 to 4
, and Comparative Examples 1 to 4
Evaluating Method / Measuring Method
[0083] The thickness of a proton exchange membrane was obtained by making a measurement using a micrometer (Mitutoyo Standard Micrometer 0-25 mm 0.01 mm). The proton exchange membrane was allowed to stand still in a measuring room wherein the room temperature and the humidity were controlled into 20° C. and 30±5 RH %, respectively, for 24 hours or more, and then the membrane was cut into a sample having a size of 5×5 cm. The thicknesses at 20 points therein were measured, and the average value thereof was defined as the film thickness.
[0084] The amount of acid-type functional groups present in an ion exchange membrane was measured as the ion exchange capacity (IEC). For sample preparation, a sample piece (5×5 cm) was first dried under the flow of nitrogen gas in an oven at 80° C. for 2 hours, and further the piece was allowed to stand still in a desiccator filled with silica gel for 30 minutes. Thereafter, the dry...
example 1
[0090] Prepared was a mixture of a disodium salt of 3,3′-disulfo-4,4′-dichlorodiphenylsulfone, 2,6-dichlorobenzonitrile, 4,4′-biphenol, and potassium carbonate to set the mol ratio therebetween into 1.00 / 5.62 / 6.62 / 7.62, and then 15 g of the mixture was weighed and put into a 100 mL four-necked flask, together with 3.50 g of a molecular sieve. Nitrogen was then caused to flow in the flask. NMP as a solvent was used. The solution was stirred at 155° C. for one hour, and then the reaction temperature was raised up to 190-200° C. The reaction was continued until the viscosity of the system was sufficiently raised (for about 4 hours). After the solution was naturally cooled, the molecular sieve, which precipitated, was removed, and a polymer was precipitated in a strand form in water. The resultant polymer was washed in boiled ultrapure water for 1 hour, and then dried. A 30% solution of the polymer in NMP was prepared. The polymer solution was cast into a thin film by casting, and dried...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com