Drug Delivery Composition Comprising a Self-Assembled Gelator
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Self-Assembled Acetaminophen Prodrugs
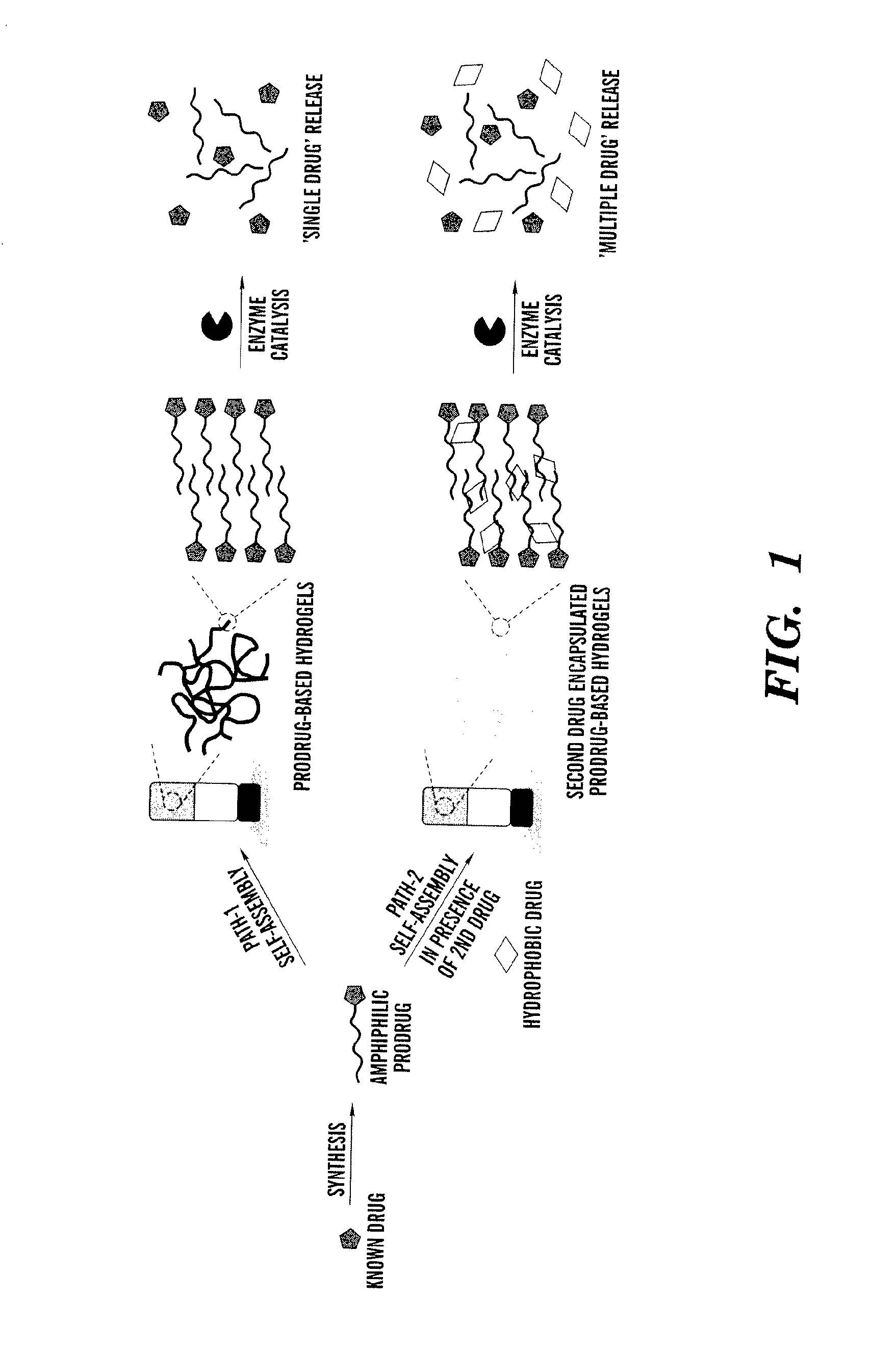
[0113]Utilization of enzyme catalysis as a tool to disassemble self-assembled hydrogels to control the release encapsulated drug provides an opportunity to employ a wide range of enzyme-specific low-molecular-weight hydrogelators.
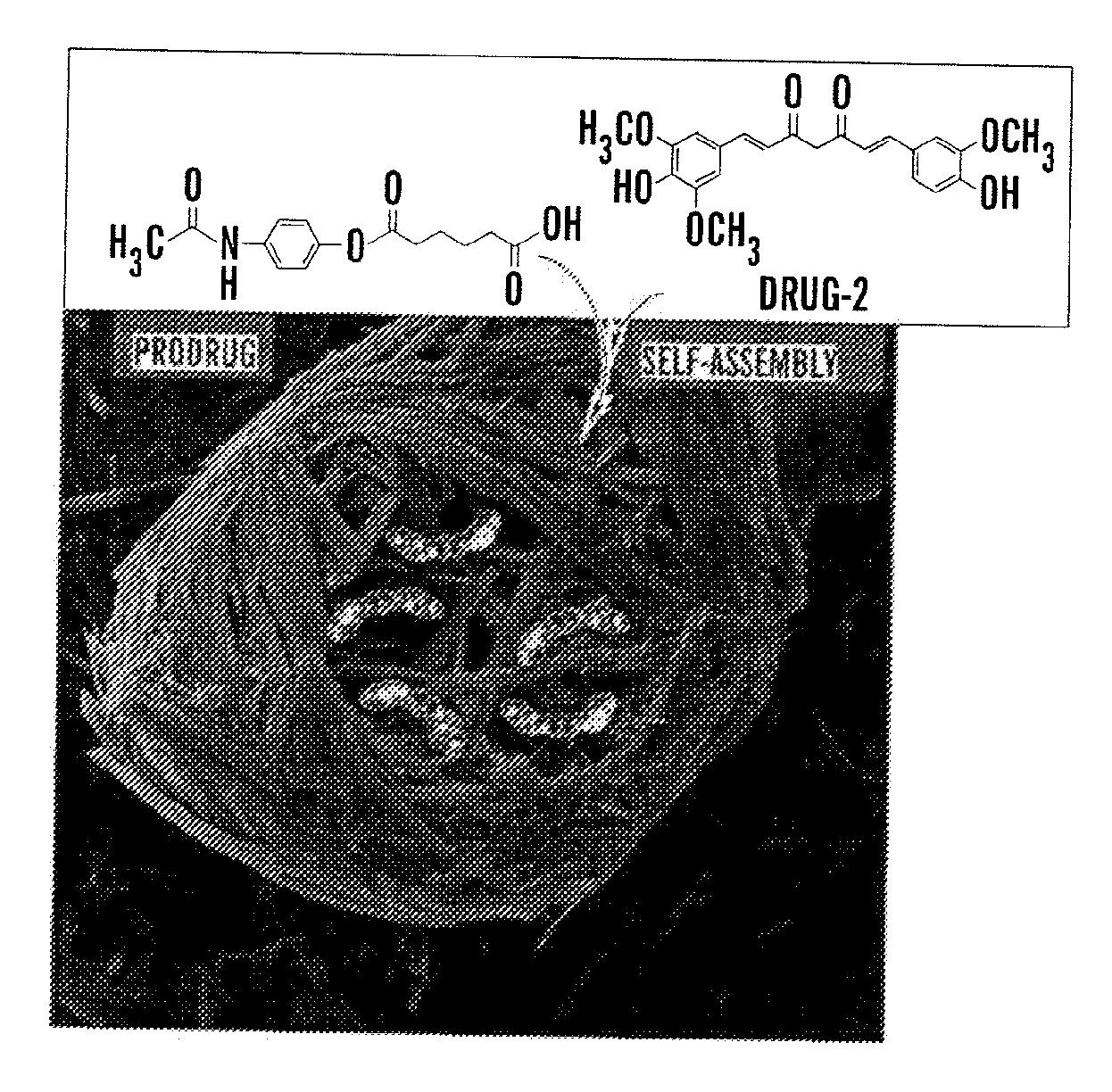
[0114]In this example, low-molecular-weight amphiphilic prodrugs are synthesized as hydrogelators from biocompatible fatty acids and a well-known drug, acetaminophen (Apn), (which belongs to a class of drugs called analgesics (pain relievers) and antipyretics (fever reducers)). This reaction was performed in a single-step esterification. The example shows the prodrug's ability to self-assemble into nanoscale structures in aqueous solutions to form hydrogels that may subsequently encapsulate a second drug such as curcumin, which is a known chemopreventive and anti-inflammatory hydrophobic drug. Upon enzyme triggered degradation, the hydrogel can release single or multiple drugs at physiologically simulated conditions in v...
example 2
Self-Assembling Amphiphiles
[0208]A series of amphiphiles were designed for efficient gelation. The amphiphile was synthesized in a single step that avoids the harsh conditions and is beneficial when synthesizing on an industrial scale. The use of expensive reagents was also eliminated. Compound purification was relatively easy. After the reaction, a simple filtration yielded a pure compound. Collectively these features make the amphiphile attractive for use in various biomedical applications.
[0209]This example focuses on a novel class of gelling agents or thickeners that are based on readily available and economically attractive starting materials. Gelling agents or thickeners capable of gelling or thickening a wide variety of solvents were used, making the gelling agents or thickeners suitable for employment in various applications.
[0210]Exemplary compounds include those having the general formula, RCONHC(CH2OH)3, in which R is a saturated alkyl chain having 9-15 C atoms or an unsa...
example 3
Dye Encapsulated Self-Assembled Nanofibers: In Vitro and In Vivo Analysis
[0230]In vitro: A model dye DiD was encapsulated within the self-assembled nanofibers prepared from salicin prodrug of formula (III). Salicin-deconate (4 wt / v %) was taken in glass vial and to that dye and PBS were added. Homogenous mixture was formed upon heating, subsequent cooling generated the gel. Fibers were isolated by repetitive centrifugation and washing steps. Fibers were incubated with synovial fluid at 37° C. that was extracted from human arthritic joints (inflamed). In the absence of synovial fluid, fibers were stable and preserved the dye within assembled fibers (FIG. 13a). The enzymes that were present in inflamed joints degraded the fibers to release the encapsulated dye. Slow degradation of fibers by enzymes over a period of 15 days was observed (FIG. 13b). The data demonstrates that in the absence of synovial fluid the fibers were intact and dye was confined to the fibers, whereas in the prese...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com