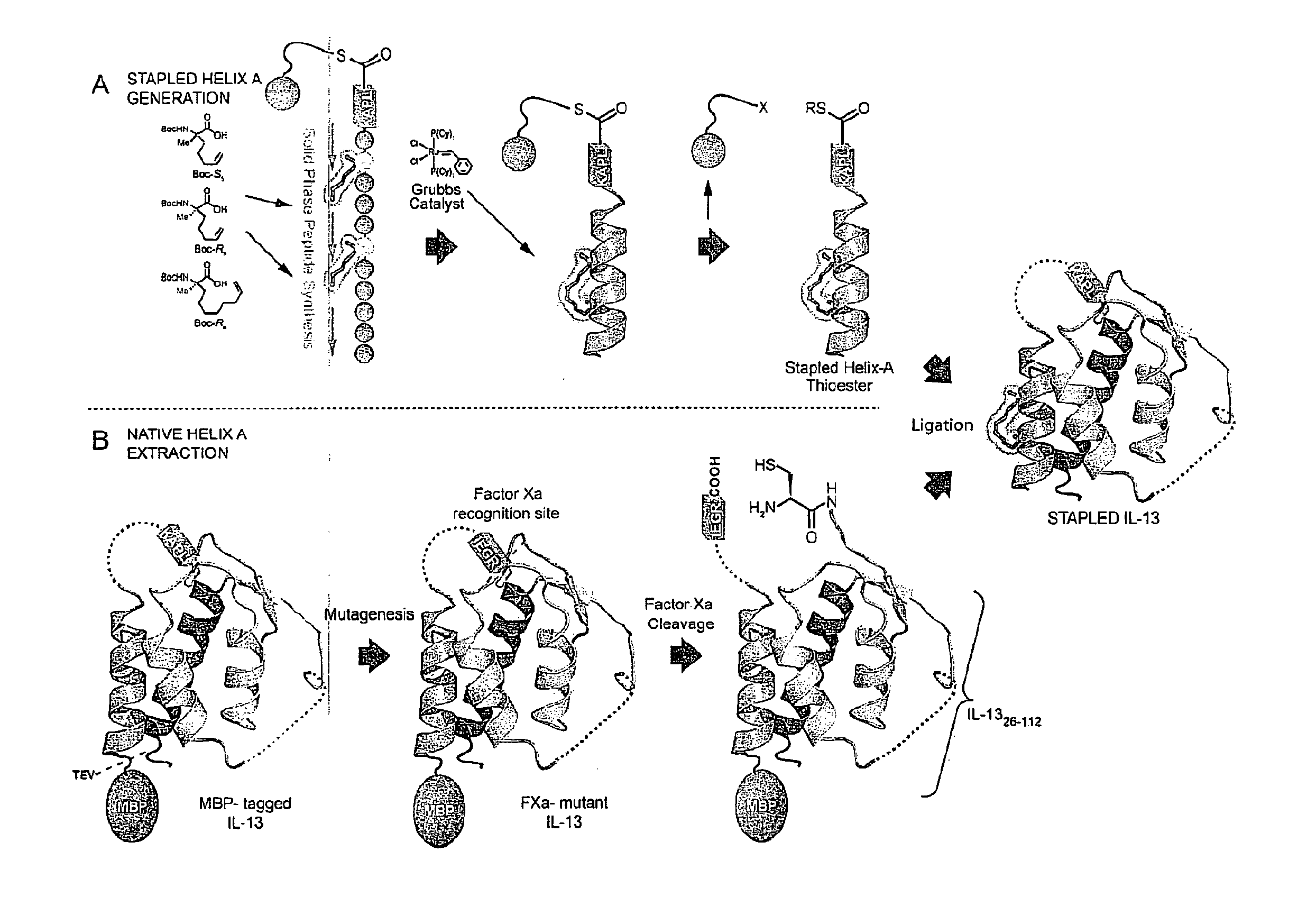
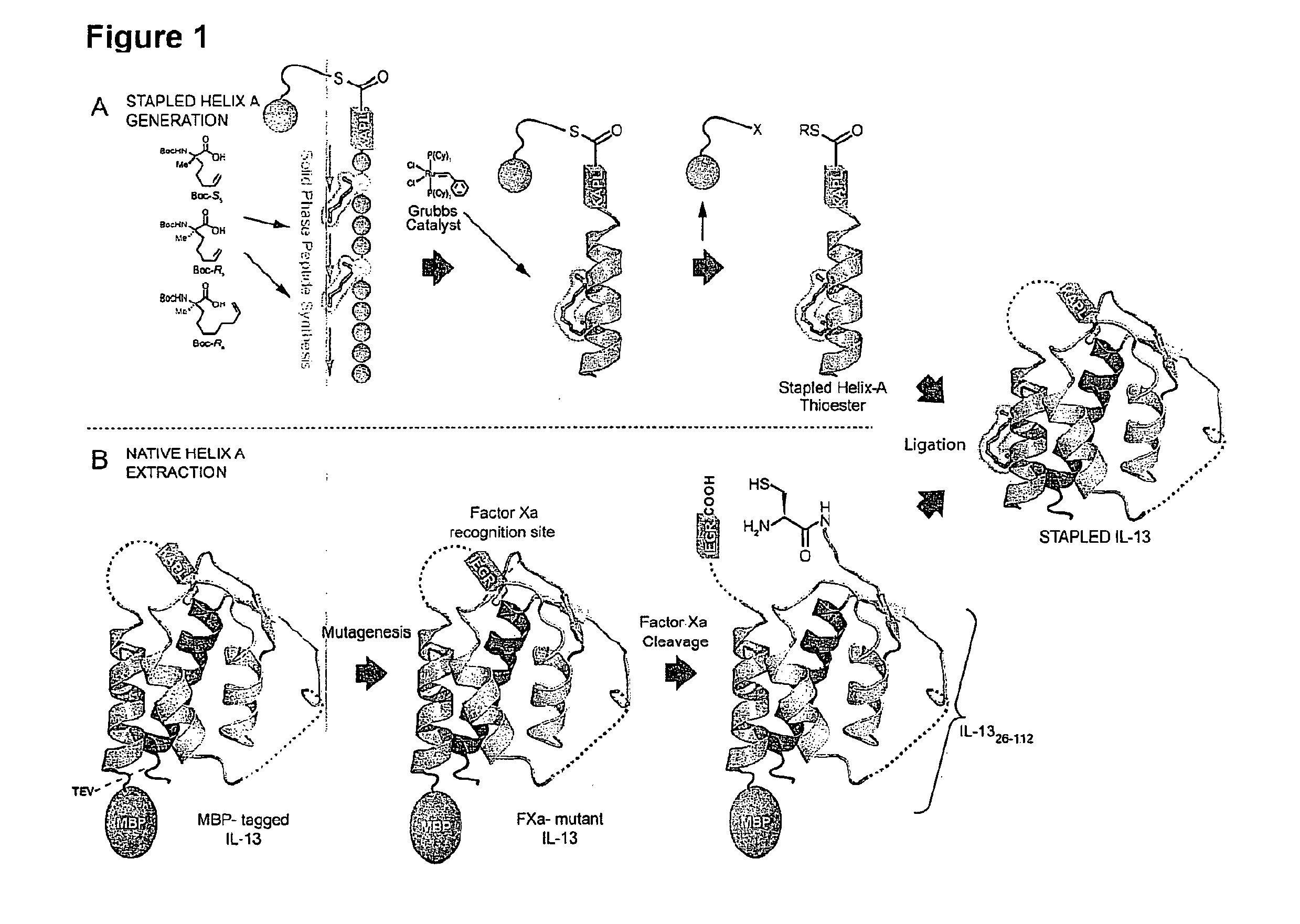
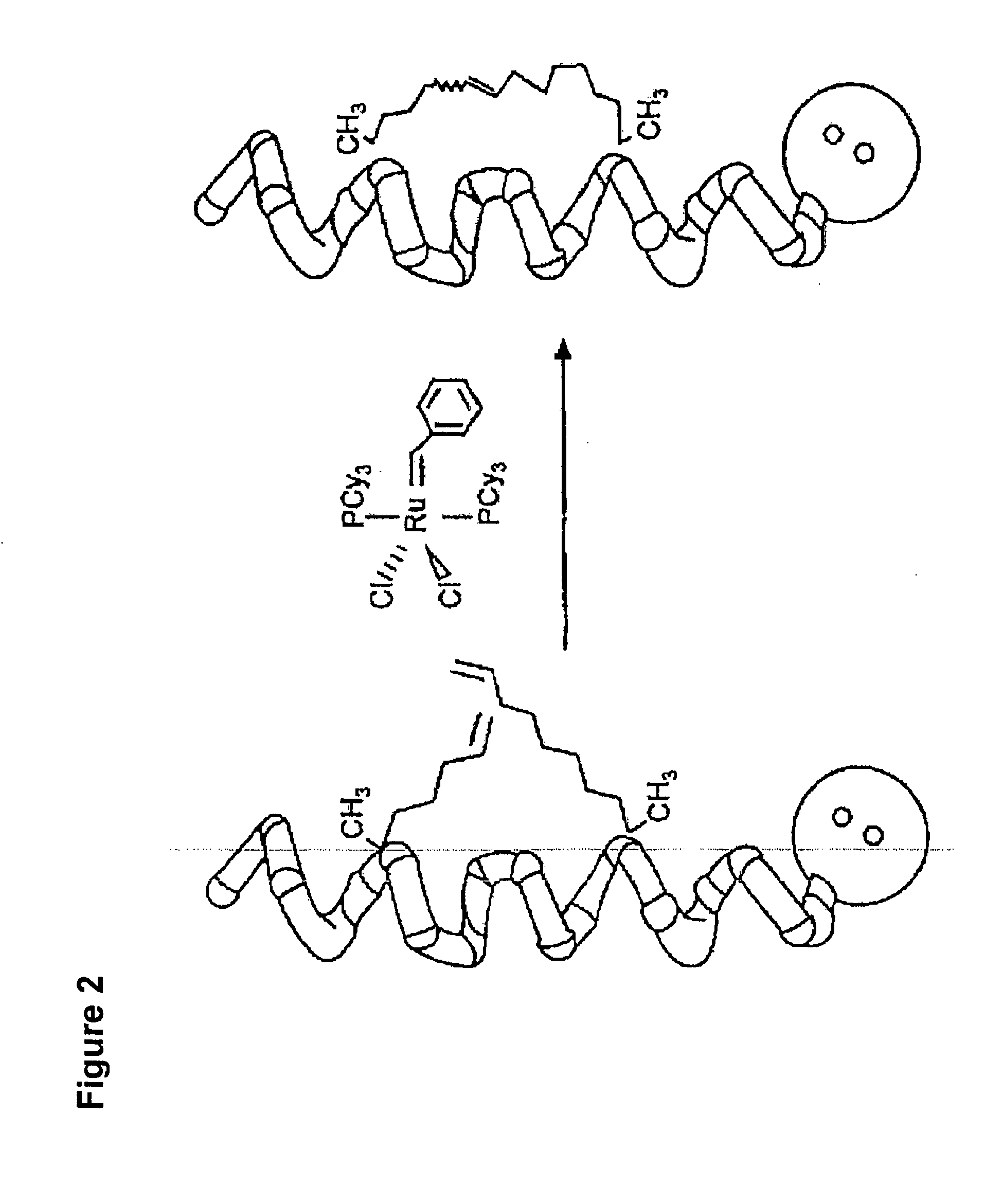
Ligation of stapled polypeptides
a polypeptide and staple technology, applied in the field of ligation of stapled polypeptides, can solve the problems of unfavorable protein production and monumental undertaking, and achieve the effects of improving activity, reducing the risk of infection, and improving the quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Semi-Synthetically Modified IL-13 Analogs
[0262]The cytokine IL-13 is strongly implicated in the pathogenesis of asthma. Interest exists in developing potent, selective, long-lasting, and non-immunogenic inhibitors of IL-13 signaling as a new therapeutic avenue in treating asthma. Using hydrocarbon stapling, IL-13 is converted from an agonist into an antagonist having the aforementioned properties through site-specific introduction of a stapled α-helix.
[0263]First, a semi-synthetic route to IL-13 analogs comprising a hydrocarbon staple on Helix-A is established. Second, a series of stapled IL-13 proteins differing in the location of the staple, their chemical composition, and stereochemical configuration is produced. Third, the binding of the stapled IL-13 analogs to IL-13Rα1 and IL-4Rα is characterized, and the structures of the relevant bound complexes is determined. Fourth, the ability of the stapled IL-13 analogs to induce or antagonize IL-13 signaling in cells is tested. Fifth, ...
example 2
Chemical Modification of the DNA Binding Basic Region of Max
[0273]Max is a member of the bHLH-LZ (basic region-helix 1-loop-helix 2-leucine zipper) family of transcription factors. While Max can homodimerize and bind to specific Enhancer box (E box) sequences, Max is also an obligate partner for several members of the Myc family for E box binding. Max is constitutively expressed and is believed to establish a basal state of transcriptional activity for target genes that are also recognized by other Myc family members. When Myc family members are upregulated, these heterodimer complexes will compete with Max homodimers for transcriptional regulation, leading to transcriptional upregulation or repression of their target genes. Myc family members are responsible for transcriptional regulation of numerous key cellular processes including cell cycle regulation, apoptosis and metabolism, and deregulated activity of Myc family members has been associated with a variety of malignancies. Max...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dihedral angles | aaaaa | aaaaa |
| dihedral angles | aaaaa | aaaaa |
| dihedral angles | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com